European Pharmacopoeia: 2.2.7. Optical Rotation
Optical rotation, as defined by the European Pharmacopoeia, is a critical physical property of chiral substances, which can rotate the plane of polarized light.
This characteristic is vital in the pharmaceutical industry as it helps in identifying the correct enantiomer of a drug, ensuring the therapeutic efficacy and safety of pharmaceuticals that can exist in chiral forms.
What is optical rotation?
Chiral molecules are characterized by their property to rotate the plane of polarized light. Therefore, they are called optically active and the effect caused by this property is referred to as optical rotation. Optically active substances may be pharmaceuticals, flavors and fragrances, amino acids, sugars, and many more biomolecules.
How optical rotation is measured with polarimetry
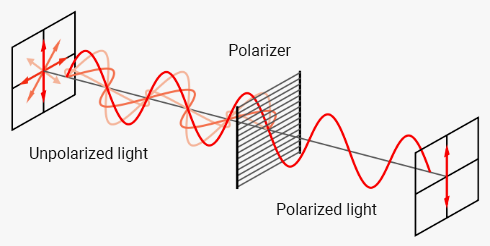
The optical rotation can be determined with polarimetry. In polarimetry, plane-polarized light is applied. Light sources, like for example a light bulb, a light-emitting diode (LED), emit electromagnetic light waves. Their electrical field oscillates in all possible planes vertical to their direction of propagation.
If this light hits a polarizer, only that part of the light may pass that oscillates in the defined plane of the polarizer. That plane is called the plane of polarization (Fig. 1).
Polarimeters
A polarimeter, the instrument used to measure optical rotation, comprises several key components:
Light source: Historically, polarimetry was performed using an instrument where the extent of optical rotation is estimated by visual matching of the intensity of split fields. For this reason, the D line of the sodium lamp at the visible wavelength of 589 nm was most often employed.
It is now common practice to use other light sources such as light-emitting diodes (LEDs) or xenon or tungsten halogen lamps, with appropriate filters, instead of traditional light sources because these contemporary light sources may offer advantages of cost, long life, and broad wavelength emission range.
Polarizer: Filters the incoming light so that only light polarized along a single plane passes through, which is then subject to rotation by the sample.
Analyzer: Aligned with the plane of polarization to measure the angle by which the plane of light has been rotated by the sample.
Sample cell: Usually made from quartz or other transparent materials, it holds the sample solution. It is designed with a path length typically about 1.00 dm, unless otherwise specified.
Detector: Captures the light after it passes through the analyzer and computes the degree of rotation.
The plane of polarization is turned by optically active compounds, the enantiomers. According to the direction in which the light is rotated, the enantiomer is referred to as dextrorotatory (d or +; Latin: right; clockwise) or levorotatory (l or -; Latin: left; counter-clockwise). The optical activity of enantiomers is additive. If different enantiomers coexist together in one solution, their optical activity adds up.
Solutions with the same concentration of both enantiomers of a chiral compound are called racemates. Racemates are optically inactive, as the clockwise and counter-clockwise optical rotations cancel each other out.
The optical rotation is proportional to the concentration of the optically active substances in solution. Polarimetry may therefore be applied for concentration measurements of enantiomer pure samples. With a known concentration of a sample, polarimetry may also be applied to determine the specific rotation (a physical property) when characterizing a new substance.
The concept of chirality and optical activity
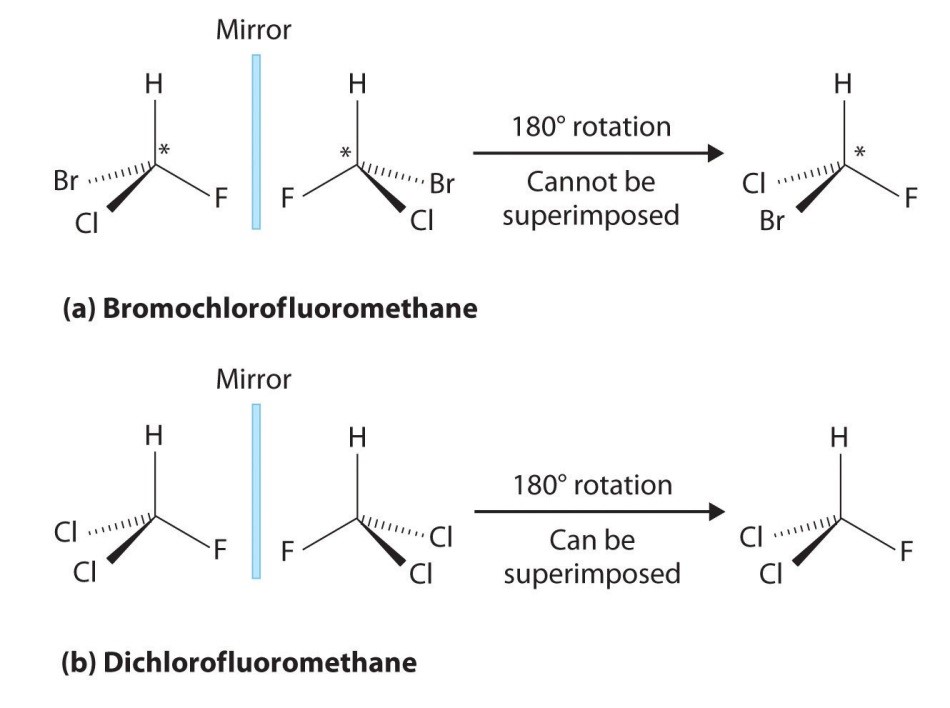
The geometrical property of a molecule that is analyzed with a polarimeter is called chirality. Chiral molecules are characterized by the mirror image constitution of their atoms. The mirror image of such a compound cannot be superimposed with the original.
The name chirality comes from the Greek cheir (hand) and reflects a molecule´s property in which its mirrored structure cannot be superimposed with the original – just like left and right hands.
The precondition for chirality within a molecule is an atom with at least four different substituents. Such an atom is called a chiral center. A practical example would be a saturated carbon: With four different substituents bound to one carbon the molecule is asymmetric. Via the exchange of two substituents at the chiral center, a molecule is created that cannot be superimposed with the original.
Most chiral substances are organic molecules, which are characterized by an asymmetric carbon. Chiral substances don’t just occur in organic chemistry but may also be made up of any kind of atom that can bind at least four different substituents, e.g. phosphor or sulfur.
What is specific rotation?
Specific rotation is a material constant. This is the optical rotation for a specific number of optically active molecules present in the light's path through the sample. The number of molecules that the light beam encounters is determined by:
- The concentration of the optically active substance, c [g/mL] → the higher it is, the more molecules there are
- The length of the sample cell l [mm] → the longer it is, the more molecules there are along the way
- The temperature, T [°C] → it influences density via thermal expansion and may cause changes in molecular structure with effects on the optical rotation
Another parameter that influences the measured optical rotation is the wavelength of the light, λ [nm].
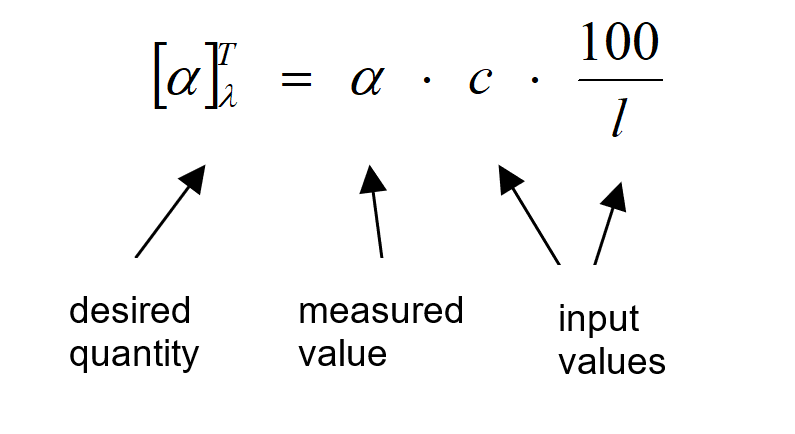
The substances’ specific rotation $[\alpha]^T_{\lambda}$ is defined as the optical rotation for:
- Concentration c = 1 g/mL
- Length of the sample cell l = 100 mm
- Temperature T = 20 °C
- Wavelength λ = 589 nm (corresponding to Nad-line)
The relationship between optical rotation, °, and specific rotation, $[\alpha]^T_{\lambda}$, is summarized by Biot’s law:
$$\alpha = \frac{[\alpha]^T_{\lambda} \cdot l \cdot c}{100}$$
Given the input parameters, the instrument works out the specific rotation automatically (select method-specific rotation).
Ph. Eur. 2.2.7. measurement technique
Standardized measurement protocols
The European Pharmacopoeia specifies standardized procedures to measure optical rotation, or specific rotation, to ensure consistent traceability across laboratories:
- Instrumentation: A polarimeter equipped with a sodium vapor lamp emitting light at the sodium D-line (589 nm) is the standard instrument. This wavelength is chosen because it is a primary spectral line and provides a consistent reference point. Instruments must also have a polarizer, an analyzer, and a sample cell typically 1.00 dm in path length unless otherwise specified.
- Setting the zero point: Before measuring optical rotation, it is crucial to determine the polarimeter's zero:
- For neat liquids: The zero is set using an empty sample cell, ensuring that the measurements reflect only the optical activity of the liquid sample without interference from other variables.
- For solutions: The zero is set using the same solvent as in the sample but without the active substance. This standardizes the baseline to account for any optical properties or impurities present in the solvent.
- Temperature and wavelength controls:
- Temperature: Measurements are conducted at a controlled temperature of 20 ± 0.5 °C. Temperature control is essential as variations can affect the density and optical properties of the sample, altering the measurement outcomes.
- Wavelength: The 589 nm sodium D-line is used for its stability and reliability in providing consistent results across different setups.
- Sample preparation: Accurate sample preparation is essential for reliable measurements. This includes the correct solvation and dilution of the sample, using the same solvent for zero setting in the polarimeter if measuring solutions. The concentration of the sample and the path length of the light through the sample are critical parameters that need to be precisely controlled and recorded.
- Documentation and compliance: Ph. Eur. mandates that all measurements be thoroughly documented, including calibration details of the instrument, sample conditions, and environmental settings during the measurement. This documentation is essential for regulatory compliance and quality assurance in pharmaceutical manufacturing.
The specific optical rotation $[\alpha]_{\lambda}^t$ is calculated using the formulas:
Neat liquids:
$$[\alpha]_{\lambda}^t = \frac{\alpha}{l \cdot \rho_t}$$
Where $\rho_t$ is the density (or relative density) at temperature t.
Solutions:
$$[\alpha]_{\lambda}^t = \frac{1000 \alpha}{l \cdot c}$$
Where c is the concentration in grams per liter.
Here:
- $\alpha$: Measured rotation angle (degrees).
- l: Path length of the sample cell (dm).
If limits for optical rotation or specific rotation are specified for the dried, anhydrous, or solvent-free substance, results must be corrected for loss on drying, water content, or solvent content, as appropriate.
Importance in pharmaceuticals
Substances requiring optical rotation testing according to international pharmacopoeias
- Tetra-O-acetyl-mannose triflate for radiopharmaceutical preparations
- Bitter-fennel fruit oil
- Bitter-fennel herb oil
- Caraway oil
- Cassia oil
- Cinnamon bark oil, Ceylon
- Cinnamon leaf oil, Ceylon
- Citronella oil
- Clary sage oil
- Clove oil
- Coriander oil
- Dwarf pine oil
- Eucalyptus oil
- Juniper oil
- Lavender oil
- Lemon oil
- Mandarin oil
- Mint oil, partly dementholised
- Neroli oil
- Niaouli oil, cineole type
- Nutmeg oil
- Peppermint oil
- Pine sylvestris oil
- Rosemary oil
- Spanish sage oil
- Spike lavender oil
- Sweet orange oil
- Tea tree oil
- Turpentine oil
- Acarbose
- Acetylcysteine
- β-Acetyldigoxin
- N-Acetyltryptophan
- N-Acetyltyrosine
- Adenosine
- Adrenaline
- Adrenaline tartrate
- Alanine
- Alcuronium chloride
- Alfadex
- Allantoin
- Alprostadil
- Amfetamine sulfate
- Amikacin
- Amikacin sulfate
- Amlodipine besilate
- Ammonium glycyrrhizate
- Amoxicillin sodium
- Amoxicillin trihydrate
- Ampicillin
- Ampicillin sodium
- Ampicillin trihydrate
- Apomorphine hydrochloride hemihydrate
- Aprepitant
- Arginine
- Arginine aspartate
- Arginine hydrochloride
- Ascorbic acid
- Ascorbyl palmitate
- Asparagine monohydrate
- Aspartame
- Aspartic acid
- Atazanavir sulfate
- Atenolol
- Atropine
- Atropine sulfate
- Azithromycin
- Bacampicillin hydrochloride
- Bambuterol hydrochloride
- Beclometasone dipropionate
- Beclometasone dipropionate monohydrate
- Benazepril hydrochloride
- Betadex
- Betamethasone
- Betamethasone acetate
- Betamethasone dipropionate
- Betamethasone sodium phosphate
- Betamethasone valerate
- Biotin
- Boldine
- Brimonidine tartrate
- Bromocriptine mesilate
- Brompheniramine maleate
- Bupivacaine hydrochloride
- Buprenorphine
- Buprenorphine hydrochloride
- Buserelin
- Cabazitaxel acetone
- Cabergoline
- Calcium ascorbate dihydrate
- Calcium folinate hydrate
- Calcium levofolinate hydrate
- Calcium pantothenate
- d-Camphor
- Camphor, racemic
- Cannabidiol
- Capecitabine
- Captopril
Relevance to quality control
Optical rotation measurements are a non-destructive, efficient way to monitor the quality of substances continuously. They are used in quality control processes to detect impurities and ensure that the correct enantiomer is present in a chiral drug. Limits for optical rotation or specific rotation, if specified for the dried, anhydrous, or solvent-free substance, must be corrected for loss on drying, water content, or solvent content, as appropriate.
Anton Paar polarimeters for optical rotation measurements in compliance with European Pharmacopoeia 2.2.7
Anton Paar polarimeters are designed to perform precise measurements of optical rotation, aligning with the stringent requirements of European Pharmacopoeia 2.2.7. These instruments are employed extensively in fields requiring accurate chiral analysis, such as pharmaceutical quality control.
Compliance with European Pharmacopoeia standards
The modular design of Anton Paar polarimeters allows them to adapt to the specific wavelength requirements mandated by European Pharmacopoeia 2.2.7. These polarimeters offer configurations that support measurements at 589 nm, the sodium D-line, which is the standard wavelength specified for optical rotation measurements. This ensures that the instruments are compliant with the pharmacopoeia's criteria and can be adjusted for future changes or additional requirements.
Precision and temperature control
Temperature control is crucial for accurate optical rotation measurements because temperature variations can significantly affect the results. Anton Paar polarimeters feature an advanced Peltier temperature control system that maintains the sample temperature within a precise range (10 °C to 45 °C), as required by the pharmacopoeia. This system allows for rapid and stable temperature adjustments, ensuring consistent and reliable measurement outcomes.
Traceability and documentation
Anton Paar polarimeters include technologies like FillingCheck™, which provides real-time visual documentation of the sample filling process. This feature is critical for detecting and correcting errors such as bubbles or streaks that could compromise the accuracy of the measurements. The instruments automatically store these images alongside the measurement results, offering detailed documentation that supports traceability and compliance with European Pharmacopoeia standards.
Data integrity and system integration
Ensuring data integrity is essential for compliance with European Pharmacopoeia standards. Anton Paar polarimeters are equipped with software that supports full data traceability, secure user management, and electronic records. The instruments are capable of integrating seamlessly with laboratory information management systems (LIMS), facilitating efficient data transfer and management. This integration supports compliance with Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP), which are often required alongside pharmacopoeia standards.
By adhering to the specific requirements of European Pharmacopoeia 2.2.7, Anton Paar polarimeters provide an effective solution for the precise measurement of optical rotation, ensuring compliance and reliability in environments where accuracy is paramount.
FAQs
1. Why is optical rotation crucial for chiral drugs?
Optical rotation is essential for chiral drugs as it identifies and characterizes enantiomers, which often differ in pharmacological activity. It ensures the presence of the correct enantiomer (eutomer) and detects impurities or racemic mixtures that could affect efficacy or safety. Optical rotation correlates with biological activity since drug-receptor interactions are stereospecific. It is a non-destructive method used in quality control, regulatory compliance, and stability testing to confirm stereochemical integrity over a drug’s shelf life. Examples like thalidomide and L-Dopa highlight its importance in verifying the therapeutic enantiomer and avoiding adverse effects associated with the distomer.
2. How does optical rotation affect drug safety?
Optical rotation ensures drug safety by confirming the presence of the correct enantiomer in chiral drugs, as enantiomers can have vastly different biological effects. The desired enantiomer (eutomer) provides therapeutic benefits, while the other (distomer) may be inactive or harmful. Optical rotation detects enantiomeric impurities or racemic mixtures that could compromise efficacy and safety. It is also critical for monitoring stereochemical stability during storage, preventing unsafe isomer formation. By ensuring compliance with pharmacopoeia standards for stereochemistry, optical rotation plays a vital role in maintaining the purity, quality, and safety of chiral drugs for patients.
3. What are the specific requirements for optical rotation measurements as per Ph. Eur. 2.2.7?
The European Pharmacopoeia (Ph. Eur.) section 2.2.7 outlines the specific requirements for optical rotation measurements to ensure accurate and standardized testing. These requirements are as follows:
- A sodium discharge lamp emitting light at 589 nm (sodium D-line) is standard.
- Alternatively, a light-emitting diode (LED) or other suitable light sources may be used, provided the wavelength is controlled.
- A polarimeter including a polarizer, analyzer, and a sample cell of 1.00 dm path length, unless specified otherwise
- Measurements must be performed at a specified temperature (typically 20 ± 0.5 °C) with a device capable of readability to 0.1 °C
- A Peltier system or external temperature control (e.g., a cycle-cryostat) is used to maintain temperature stability
References
- European Pharmacopeia chapter 2.2.7.
- Bruice, P. (2011). Organische Chemie.7th Ed. Santa Barbara. Pearson, pp.191-192.
- Averill, B. and Eldredge, P. (2011). General Chemistry: Principles, Patterns, and Applications. Washington, D.C. Saylor Foundation, p. 1099.