Clay Applications and Characterization
What is clay?
Clay application spans multiple industries due to its unique properties. It is a natural, fine-grained earthy substance composed mainly of tiny mineral particles and organic matter. It forms through the weathering and decomposition of rocks, particularly granite and feldspar-rich rocks such as basalt. Humans have utilized clay for various purposes for millennia, owing to its distinctive properties. Its properties, such as plasticity, cohesiveness, and absorbency, make it useful in various fields, including construction, agriculture, art, and cosmetics. The diverse characteristics of different types of clay enable them to be customized for specific uses and applications. Here are some key characteristics of clay:
Particle size: Clay particles are extremely small, typically less than 2 µm in diameter. This fine particle size contributes to the unique properties of clay.
Mineral composition: Clay is primarily composed of various clay minerals, with the most common being kaolinite, illite, and smectite. These minerals give different types of clay their distinct characteristics.
Plasticity: One of the most notable properties of clay is its plasticity, which allows it to be easily molded when mixed with water. This property is what makes clay suitable for shaping and sculpting into various forms.
Absorption and retention: Clay has the ability to absorb and retain water. This property is often utilized in agriculture for improving soil moisture retention and in cosmetics for oil and impurity absorption.
Cohesiveness: When moistened, clay particles can stick together and create a cohesive mass. This makes clay suitable for building materials like bricks, tiles, and pottery.
Color variation: Clay can come in a range of colors, including white, red, brown, green, and gray. The color of clay is influenced by the mineral content and impurities present.
Firing characteristics: Clay can be hardened and made durable by firing it at high temperatures in a kiln. This process is commonly used in ceramics and pottery production.
Versatile uses: Clay has a wide range of applications, including pottery, ceramics, bricks, tiles, construction materials, cosmetics, pharmaceuticals, agriculture, and even as a medium for artists and sculptors.
Environmental importance: Clay plays a role in the environment by contributing to soil composition. It can influence the fertility, structure, and water-holding capacity of soils, which is crucial for plant growth.
Clay structure and classification
Clay minerals, like other phyllosilicates, have special structures made up of flat sheets. These sheets are built from shared tetrahedra (a shape with four triangular faces) and octahedra (a shape with eight faces). In the case of clay, these sheets are made up of aluminum and silicon atoms, and they have a chemical formula of (Al, Si)3O4.
Imagine these sheets as flat layers where each triangle shares three of its corners with other triangles, forming a hexagon in two dimensions. All the triangles in these layers point in the same direction, and the unshared corner of each triangle is on one side of the sheet. This unshared corner is called an apical oxygen ion. In clay minerals, these tetrahedral sheets are always connected to octahedral sheets, which are made from smaller ions like aluminum or magnesium. These octahedral sheets are coordinated by six oxygen atoms. The unshared corner from the tetrahedral sheet is also part of one side of the octahedral sheet. Additionally, there is an extra oxygen atom above the gap in the tetrahedral sheet, and it is connected to a hydrogen atom, forming an OH group in the clay's structure.
Clays can be classified based on how these tetrahedral and octahedral sheets are arranged in layers. If there's only one tetrahedral and one octahedral group in each layer, it's called a 1:1 clay. On the other hand, a 2:1 clay has two tetrahedral sheets with their unshared corners facing each other, forming each side of the octahedral sheet.[1]
Clay minerals can be grouped into several categories based on their composition and structure. Here are some of the main groups:
- The kaolin group contains minerals such as kaolinite, dickite, halloysite, and nacrite and is composed of polymorphs of Al2Si2O5(OH)4. Some sources also include the kaolinite-serpentine group due to structural similarities.
- The smectite group is divided into dioctahedral smectites, including minerals like montmorillonite, nontronite, and beidellite, and trioctahedral smectites, including minerals like saponite. Smectite clay minerals were even discovered on Mars based on analytical tests by the Curiosity rover in 2013.
- The illite group consists of clay-micas; illite is the sole common mineral in this group.
- The chlorite group encompasses a wide range of similar minerals with notable chemical variation.
Apart from the groups already mentioned above, there are many others such as palygorskites or sepiolites.
Clay minerals are a diverse group of different minerals, each with its own unique characteristics and applications. These minerals play important roles in various natural and industrial processes.[2]
Clay applications
Understanding and controlling the particle size and stability of clay is crucial for tailoring its properties to specific applications. Different types of clay, each with varying particle sizes, can be selected based on the desired characteristics for particular uses, making clay a versatile material across industries such as construction, agriculture, ceramics, and more. The particle size and zeta potential of clay are key characteristics with significant implications for various industries and applications.
Moreover, ongoing innovations in technology and manufacturing continue to broaden the scope of clay applications, revealing new possibilities in advanced materials, environmental solutions, and beyond.
Clay characterization
Particle size analysis and zeta potential analysis are essential tools for characterizing clay materials used in construction applications such as bricks, ceramics, and concrete. Particle size analysis ensures uniformity and quality by controlling the particle size distribution, which is vital for maintaining structural integrity and performance. Meanwhile, zeta potential analysis provides crucial insights into the electrostatic behavior of colloidal systems, aiding in understanding dispersion and stability, flocculation control, filterability, colloidal behavior, and clay mineral characterization.
Applications of clay in ceramics and pottery
Clay materials are essential for the production of ceramics and pottery. Particle size analysis helps potters and ceramicists achieve the desired characteristics in their products, such as strength, porosity, and glaze quality. The ceramics industry benefits from zeta potential analysis by guiding the selection and treatment of clays to achieve desired properties in the final product. This ensures stability during the manufacturing process, resulting in high-quality ceramic and building materials.
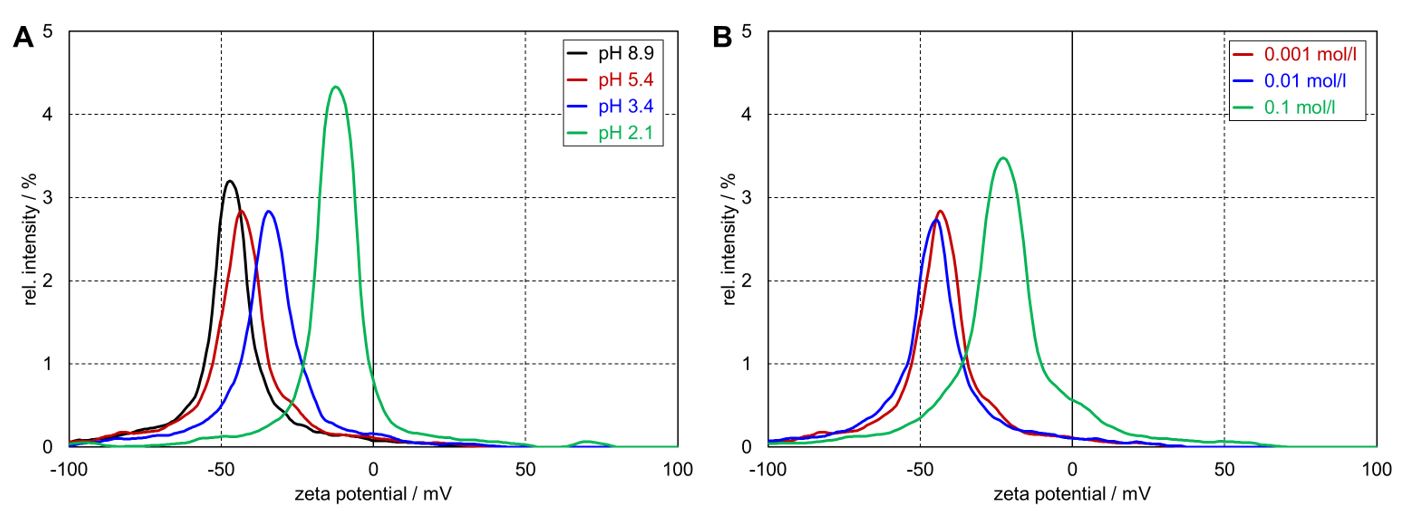
Figure 1 depicts different zeta potential distributions of kaolin powders dispersed at different pH values and different ionic strengths of aqueous KCl solution. For the application of kaolin as a filler material, preparing a homogeneous particle dispersion with predictable stability requires knowledge of the average charge density, represented by the zeta potential. Electrophoretic light scattering (ELS) is a dependable and precise method for this measurement.
Quality control in construction and building materials
Particle size analysis is critical for ensuring the consistency and quality of clay materials used in construction applications like bricks, ceramics, and concrete. Controlling particle size distribution is vital for maintaining the structural integrity and performance of these materials.
Additionally, the industry benefits from zeta potential analysis, which guides the selection and treatment of clays to achieve desired properties in the final product. This ensures stability during the manufacturing process, resulting in high-quality building materials.
Pharmaceutical industry and cosmetics
Nanoscale clay materials are valuable in pharmaceuticals and cosmetics as excipients and APIs. Chemically modified nanoclays (<100 nm) show promise in drug delivery, gene delivery, and bioimaging. Particle size analysis helps tailor their properties, while zeta potential influences interactions with biological systems, drug release, and therapeutic efficiency. Zeta potential is crucial for assessing product quality, stability, and shelf life and is extensively used in quality control and formulation of liquid dispersions, as well as in new product development. In this context, it is important to determine particle size and zeta potential.
Wastewater
Clays play a crucial role in waste management by adsorbing contaminants, improving water clarity, and enhancing treatment efficiency. Their natural abundance, low cost, high surface area, cation exchange capacity, and adsorptive properties make them invaluable for maintaining water quality. Understanding particle size distribution is vital for designing containment systems, assessing contaminant migration, and planning remediation. Controlling zeta potential further optimizes the movement and removal of clay particles and contaminants.
Mining and mineral processing
The mining industry leverages the unique properties of various clays to enhance efficiency, safety, and environmental protection in their operations. Bentonite, kaolin, illite, and montmorillonite are the most commonly used clays, with applications ranging from drilling fluids and pelletizing to casting, environmental protection, and water treatment. These versatile materials contribute significantly to the overall sustainability and effectiveness of mining processes. Understanding particle size distribution is crucial for efficient processing and separation. Measuring zeta potential in the mining and minerals industry is essential for optimizing processes, enhancing particle stability, controlling flocculation, and improving overall efficiency. It contributes to the effective management of mineral slurries and assists in achieving desired outcomes in various mineral processing steps.
Conclusion
In conclusion, the applications of clay materials span a diverse array of industries and scientific fields, underscoring their versatility and significance. The growing importance of clay applications further highlights how this natural resource can be leveraged to meet modern demands. Particle size analysis is a crucial aspect, providing essential insights that enhance product quality and process efficiency. By understanding the particle size distribution, industries can optimize the use of clay materials to meet specific requirements, ensuring better performance and sustainability. Similarly, zeta potential analysis is proving to be a powerful tool in understanding and controlling the behavior of clay materials. This analytical method is instrumental in optimizing industrial processes and advancing scientific knowledge. The insights obtained from zeta potential analysis contribute to improved environmental management and the development of innovative materials and products. Together, particle size and zeta potential analyses form a robust foundation for leveraging the full potential of clay materials in various applications, paving the way for more efficient and effective industrial and scientific endeavors.
Future research into novel clay applications likewise promises to address emerging global challenges, particularly in environmental remediation, biomedical engineering, and cutting-edge industrial technologies.
References and further Reading
[1] Nesse, William D. (2000). Introduction to mineralogy. New York: Oxford University Press. pp. 235–237. ISBN 9780195106916.
[2] "The Clay Mineral Group". Amethyst Galleries. 1996. Archived from the original on 27 December 2005. Retrieved 22 February 2007.
https://www.anton-paar.com/corp-en/services-support/document-finder/application-reports/your-concrete-answer-size-and-shape-analysis-of-cement/