Dynamic Aspects of Streaming Potential
Introduction & theory
Zeta potential is a key parameter in colloid science and electrochemistry. It refers to the electrical potential at the shear plane (the interface between the solid surface and the surrounding liquid) of a colloidal particle or solid surface. When a material comes into contact with a liquid, the functional groups present on its surface undergo reactions with the surrounding medium. This interaction induces a surface charge, which in turn attracts oppositely-charged ions from the medium. Counter ions spontaneously organize into an electrochemical double layer on the surface. The zeta potential is a result of the distribution of charges from the surrounding liquid on the material's surface and the interactions with the ions in the surrounding liquid.
The zeta potential can be determined using different techniques, depending on the sample’s nature and size, such as measurement of the zeta potential using electrophoretic light scattering (ELS) or measurement of the streaming potential. If the streaming potential is used to determine the zeta potential, not only the surface zeta potential of the solid sample can be analyzed, but also information about adsorption kinetics can be gathered. Therefore, the extension of the conventional streaming potential - the so-called dynamic streaming potential - can be used.
This article introduces the dynamic streaming potential, elaborates its distinctions from the classic streaming potential, and explains a wide range of different applications, in which the dynamic streaming potential offers new possibilities.
Classic streaming potential
Streaming potential is a phenomenon that occurs in fluid-filled capillaries or porous materials when there is relative motion between the fluid and the solid surfaces.
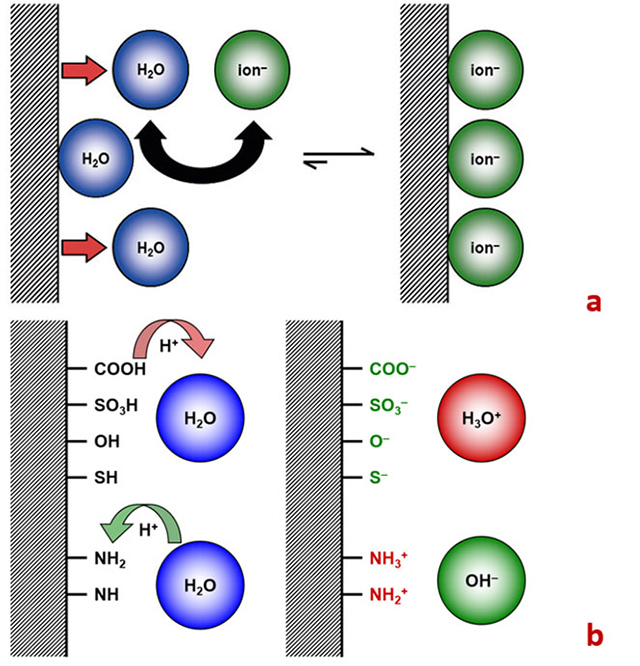
When a solid material or particle comes into contact with a liquid, the functional groups on its surface react with the surrounding medium. Two critical processes occur: the adsorption of water ions onto the surface (Figure 1a) and the chemical interactions between the surface's functional (acidic and basic) groups (Figure 1b) and the liquid medium.
These interactions lead to the development of a surface charge, which subsequently attracts oppositely-charged ions from the surrounding liquid. These counter ions attach in a layer, known as the electrochemical double layer, on the surface or around the particle. The zeta potential represents the cumulative effect of the initial surface charge and the assembled counter ion layer.[1]
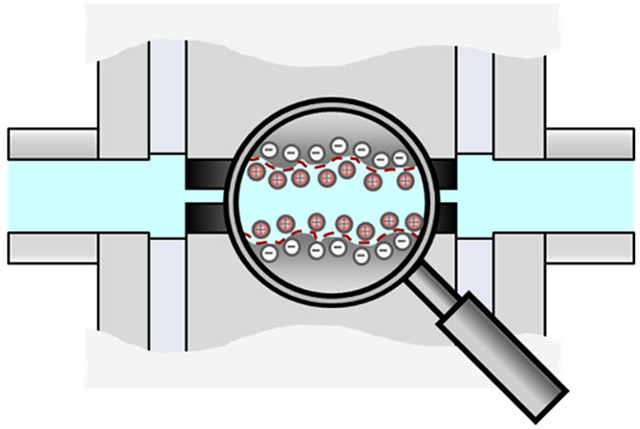
To assess the streaming potential, the solid sample is positioned within a suitable measurement cell in the SurPASS 3 instrument, creating a capillary channel with the sample pieces. Subsequently, the channel is filled with an aqueous electrolyte solution, typically a diluted salt solution. Charges develop at the interface between the solid sample and the electrolyte solution through acid-base reactions on surfaces containing functional groups (Figure 2).
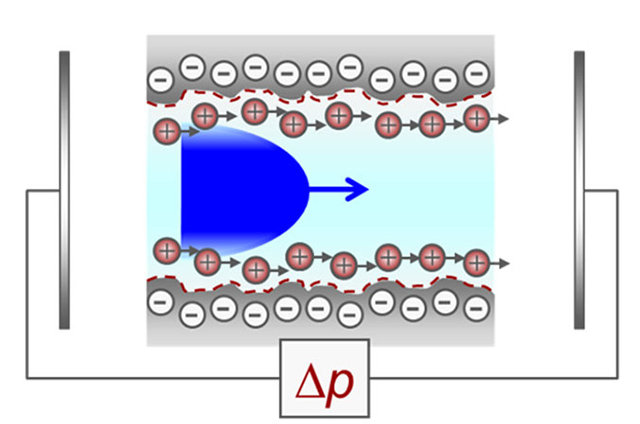
When the measurement is started, a pressure gradient is applied to the electrolyte solution, inducing a flow through the capillary in the measurement cell, as shown in Figure 3. This movement causes the charges at the liquid/solid interface to travel with the flow, resulting in an accumulation of charge carriers on one side of the measuring cell and a depletion on the opposite side (Figure 4).
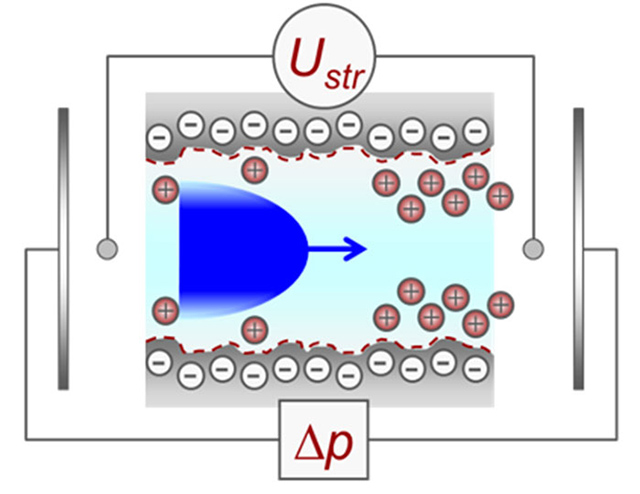
The measured streaming potential, together with the applied pressure, is used to calculate the surface zeta potential of the sample. The latter is derived from the streaming potential using equation 1 where Ustr is the streaming potential, Δp is the pressure difference across the sample, ε_o is the vacuum permittivity, ε_r, η and B are the dielectric constant, the electrolyte viscosity and conductivity, respectively.
$$\zeta = -\frac{dU}{d\Delta p} \frac{\eta k_B}{\epsilon_r \epsilon_0}$$
Equation 1
The formation of surface charge through acid-base reactions, a primary mechanism generating zeta potential, is highly dependent on the pH of the aqueous solution. Therefore, recording the pH dependence of the zeta potential is a common practice to understand the nature of functional surface groups and to determine the isoelectric point (the pH at which the zeta potential of the sample is zero).
When adjusting the pH by introducing different chemical compounds (e.g., surfactants, polyelectrolytes, polypeptides, proteins), the interactions between these compounds and the material’s surface can be investigated. Attractive forces, such as electrostatic and hydrophobic interactions, cause these dissolved compounds to adsorb onto the solid surface.
Adsorption processes
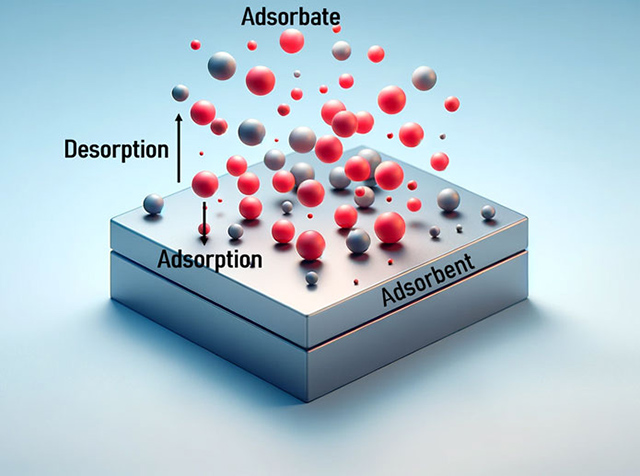
Adsorption refers to the adherence of atoms, ions, or molecules (solutes) from a gas, liquid, or dissolved solid onto a surface, forming a film of the adsorbate on the adsorbent's surface. It is a process in which molecules or particles adhere to a solid surface or liquid material, shown in Figure 5. It is a fundamental phenomenon in numerous fields, including chemistry, physics, engineering, and environmental technology.
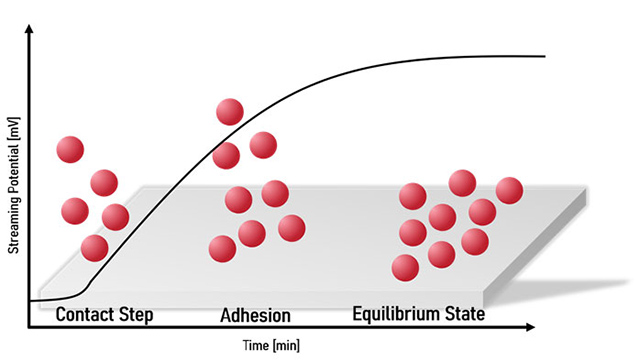
It plays a key role in numerous technical applications. The adsorption process can consist of several steps, depending on the type of adsorbent, the adsorbate and the specific conditions. Typically, this process consists of the contact step where the adsorbate reaches the adsorbent surface, the process of bond formation between adsorbate and adsorbent, and finally the equilibrium state where no further adsorption takes place. This process is depicted in Figure 6.
It is important to understand that the transition from one state to the next takes time. In particular, the time taken to reach adsorption equilibrium depends strongly on the specific conditions and is of great importance for the design of adsorption systems.
Adsorption isotherms
An important concept in this context is the adsorption isotherm. It describes how substances adhere to a surface at a constant temperature (the term "isotherm" is derived from the Greek words "ísos" meaning "equal" and "thérmē" meaning "heat".)[2] These isotherms can be derived from streaming potential measurements. The significance of adsorption isotherms lies in their ability to mathematically characterize the adsorption process and predict behavior in the equilibrium state; that means they help describe and predict how substances will adhere to a surface when an equilibrium state is reached at a constant temperature.
A closer look reveals that adsorption isotherms provide a quantitative depiction of how adsorption changes in response to variations in the concentration of the adsorbate within the system. This contributes to understanding the fundamental mechanisms and dynamics controlling the adsorption process. By analyzing adsorption isotherms, one can predict the adsorption capacity of a material at equilibrium under various conditions (e.g. changes in pH value or ionic strength of the electrolyte solution). This insight is beneficial in optimizing processes to achieve desired levels of adsorption. Additionally, adsorption isotherms are used to characterize adsorbent materials, facilitating the assessment of their properties. Since different materials may exhibit unique adsorption behaviors, isotherms play a crucial role in evaluating their suitability for specific applications.
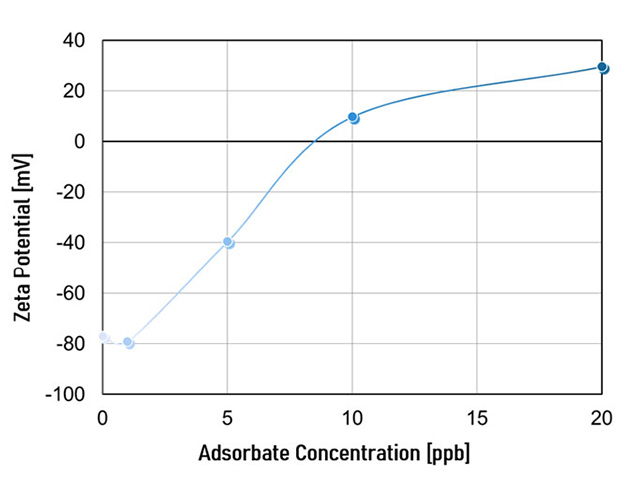
Figure 7 displays the adsorption isotherm obtained from the observed adsorption kinetics, illustrating how the adsorption process changes over time and is influenced by the zeta potential's response to changes in adsorbate concentration. As the concentration of the adsorbate in the electrolyte solution increases, the negative zeta potential of the adsorbent (e.g. a surface) decreases until charge reversal occurs. The temperature is kept constant throughout the entire measurement.
Adsorption kinetics
temperature, pressure, and adsorbate concentration influence this process. The kinetics of adsorption are often dictated by the rate-limiting steps, which are the stages that decelerate the overall process, e.g. the diffusion of the adsorbate through the liquid phase to the adsorbent’s surface, diffusion within the adsorbent's pores, or the actual formation of bonds between the adsorbate and the adsorbent. Understanding the kinetics is crucial for various physical, biological, and chemical systems, and has broad applications in industrial processes.
Conversely, desorption refers to the process by which atoms or molecules detach from the surface of a solid, effectively releasing the adsorbed substance.
Dynamic streaming potential
Dynamic streaming potential extends the classic streaming potential measurement by enabling real-time monitoring of interactions between solutes and solid surfaces. This method does not primarily focus on static processes, but emphasizes temporal changes, focusing on phenomena such as the accumulation of dissolved substances and the effects of adsorption and desorption on solid surfaces.
The principle is straightforward: an adsorbate is added to the electrolyte solution, and the resulting changes in the streaming potential are recorded over time.
If the adsorption of substances on a surface is reversible, desorption can be achieved by exchanging the liquids, allowing adsorbed substances to detach from the surface and restore the initial state of the solid surface. In assessing the dynamic streaming potential, the primary focus is on monitoring changes in charge throughout the measurement.
This change does not necessarily mean that the sign of the zeta potential changes, but rather a change in the net charge of the solid surface. This change can be examined by adjusting various factors, such as the composition or change of the pH value of the electrolyte solution.
Applications
The phenomena of adsorption and desorption play essential roles in various aspects of daily life, spanning activities such as textile laundry, hair washing, and applications within the medical and life sciences. While certain applications benefit from the desirable adsorption of substances onto surfaces, others necessitate its avoidance. Consequently, the evaluation of the dynamic streaming potential proves valuable across numerous industries for examining the adsorption kinetics of substances on various solid surfaces.
Cosmetics
Due to the diversity of hair types, trends, and other factors influencing hair health, hair care is an interesting field of application.
The development of versatile and, hence, complex formulations necessitates a thorough understanding of how hair care products directly affect hair texture. Investigating the surface zeta potential at the interface between hair fibers and an aqueous emulsion of the hair care product is crucial for understanding how formulation components interact with the hair surface, enabling precise adjustments to the formulation’s composition.
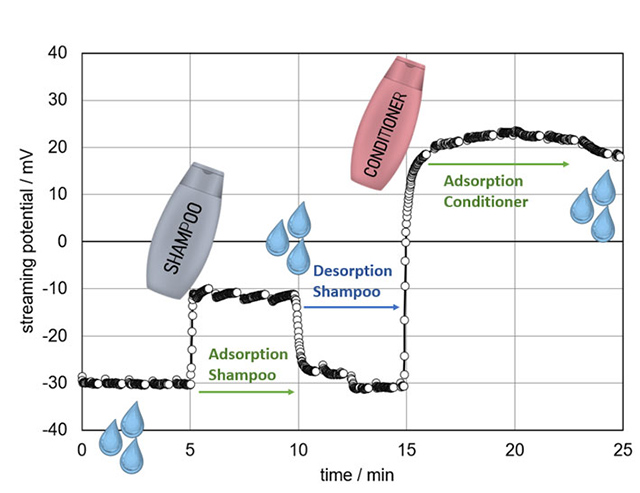
Figure 8 illustrates a typical hair washing cycle. In this experiment, a portion of a tress of Caucasian hair was first rinsed with an aqueous buffer solution and then treated with shampoo. The shampoo was rinsed out before a conditioner was applied.
Initially, the untreated hair exhibits a negative zeta potential. The application of shampoo reduces the magnitude of this negative zeta potential. Rinsing the shampoo results in the zeta potential returning to its original values, indicating that the hair was already clean prior to shampoo application. Upon the application of the conditioner, the zeta potential reverses sign, indicating the adsorption of the conditioner. After rinsing the conditioner, the zeta potential decreases in magnitude but remains positive, suggesting that some of the conditioner becomes irreversibly bound to the hair surface, while the excess is removed during rinsing.
In this context, dynamic streaming potential analysis, also in combination with the determination of the zeta potential using electrophoretic light scattering (ELS), is very insightful. By determining the zeta potential of the liquid formulation and the dynamic streaming potential when applying it onto human hair, the cosmetic chemist gets an overall picture of the stability of the products and its interaction with hair structure during application.
Textile care
Adsorption processes are integral in textile care, as well as in cleaning and stain removal. Adsorption involves the adherence of molecules or particles onto the surface of a solid material, a principle utilized in various textile care processes such as stain removal, fabric softening, and the application of specific functionalizing materials.
Detergents and stain removers contain surfactants, enzymes and other chemicals that facilitate the desorption of dirt, oils, and other stain components. These cleaning agents surround stain particles, increasing their water solubility and enabling easier removal during washing. Fabric softeners employ cationic surfactants that adsorb onto the fabric's surface. These positively-charged molecules reduce friction between fibers, resulting in a softer and smoother fabric. Additionally, they impart antistatic properties and enhance the overall texture and skin feel of the textile.
By determining the zeta potential via ELS of the detergent or fabric softener combined with the determination of the dynamic streaming potential, a large overall picture is obtained in order to understand the complete application in the entirety of the components.
Protein adsorption
The research field of protein adsorption on surfaces appears to be as popular as ever. It refers to the process by which proteins adhere to the surface of a material or interface. The adsorption of proteins onto surfaces is a dynamic and complex process influenced by various factors, including the physicochemical properties of the material, the nature of the proteins, and the surrounding environment. Proteins can undergo conformational changes upon adsorption, and the extent and pattern of adsorption can impact the performance and biocompatibility of the material.
In medical applications, understanding protein adsorption is crucial for the development of biomaterials, implants, and medical devices. The interaction between proteins and surfaces can influence cell adhesion, tissue response, and the overall biocompatibility of the material.
Protein adsorption is often studied to design surfaces that either promote or inhibit protein binding, depending on the intended application. Surface modifications, coatings, and biomimetic approaches are employed to control protein adsorption and enhance the functionality of materials in various biomedical and industrial settings, such as biomaterials (e.g. medical implants).
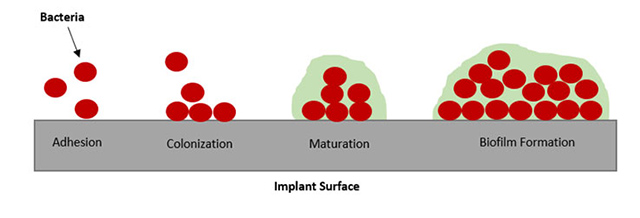
Figure 9 shows the formation of a biofilm on an implant surface. In biofilm formation, microorganisms adhere to surfaces and create a matrix of extracellular polymeric substances (EPS). Protein adsorption plays a crucial role in the initial stages of biofilm development. Proteins in the surrounding environment adsorb onto surfaces, forming a conditioning film that promotes microbial attachment. The adsorbed proteins also contribute to the formation of the EPS matrix, providing structural support for the biofilm. Controlling protein adsorption is a strategy to prevent or disrupt biofilm formation, with implications for fields such as medicine where minimizing biofilm on medical devices is important for preventing infections.
The compatibility of implant materials like stainless steel or titanium with the human body relies on the implant surface's biocompatibility. The biocompatibility is influenced by various surface attributes. The charge on the surface governs the electrostatic attractions that are crucial for bonding proteins, necessary for integrating materials like dental implants into bone, the so-called “osseo-integration”. Meanwhile, the ability to repel certain electrostatic interactions helps avoid the attachment of proteins that may cause infections. Thus, a deep understanding of these surface characteristics is essential in creating and evaluating biomaterials for implants.
Conclusion
In this article, we introduce an extension of the classic streaming potential measurement, the so-called dynamic streaming potential. This method significantly helps us understand adsorption processes at the solid-water interface.
We describe the measurement of the dynamic streaming potential and its differences to the conventional streaming potential. This method for zeta potential analysis and the recording of adsorption isotherms gives a better understanding of adsorption phenomena at the solid-water interface. This enhanced perspective is important in guiding the optimization and refinement of products across these domains, ensuring their improved performance and efficacy within different applications.
Further Literature
- Relevance of zeta potential analysis for textiles
- Zeta potential studies on the surface treatment of prefilled syringe barrels
- Race for the surface: Surface zeta potential analysis of implants
- Webinar: The beauty and the zeta potential - Determine formulation stability and behavior of cosmetic products
- Dynamic streaming potential analysis Episode 1: Application in textile care
- Dynamic streaming potential analysis Episode 2: Application to medical devices
- Dynamic streaming potential: Basics and applications
References
[1] Luxbacher, Thomas. The ZETA Guide . Graz : Anton Paar GmbH, 2014.
[2] Gemoll, Wilhelm. Griechisch-Deutsches Schul- und Handwörterbuch. Munich/Vienna : G. Freytag Verlag/Hölder-Pichler-Tempsky, 1965