European Pharmacopoeia: 2.2.6. Refractive Index
Refractive index, as described in the European Pharmacopoeia, represents the ratio of the phase velocity of light in a vacuum to its velocity in a given medium. This measure is crucial in various fields including materials science and pharmaceuticals, where it aids in the identification, quality control, and purity assessment of substances.
Understanding refractive index
The refractive index of a transparent optical medium, also referred to as the index of refraction, represents the ratio between the phase velocity and the velocity of light in a vacuum, when the optical medium has interacted with electromagnetic radiation. For transparent materials, the refractive index is a dimensionless quantity greater than 1.
The refractive index is measured for a number of different reasons. The refractive index is a relevant parameter in the context of materials science. Concentration determination of various binary solutions can be performed by means of refractometry. Furthermore, the purity of chemical and pharmaceutical ingredients can be determined.
Relevance in the European Pharmacopoeia (Ph. Eur.)
Since every substance has a characteristic refractive index under specific conditions, the measurement of refractive index in the pharmaceutical environment focuses on identification, quality control, and purity assessment of substances in pharmaceutical formulations.
Section 2.2.6 of the European Pharmacopeia states that the refractive index is determined at a temperature of 20 ± 0.5 °C and a wavelength of the sodium D-line (λ = 589.3 nm). The result is expressed with the symbol $n \frac{20}{D}$ . Any potential deviations will be outlined in the relevant monograph. Instruments have to be calibrated using certified reference materials and must give readings accurate to at least the third decimal place. Devices using white lights require a compensating system, respectively. The apparatus is operated at the prescribed temperature with a resolution of ≤ 0.5 °C.
The science behind refractive index
The refractive index of a medium is a measure for how much the speed of light is reduced inside the medium relative to the speed in a vacuum. Standard conditions are 20 °C, 1,013 mbar, and 50 % relative humidity.
Examples for speed of light in different media:
- In vacuum: 299,792 km/s
- In air: 299,710 km/s
- In water: 225,000 km/s
- In sapphire: 170,000 km/s
The higher the optical density of the media is, the lower the speed of light inside the media.
The refractive index is expressed as the ratio of the speed of light in the vacuum $v_{\text{vacuum}}$ relative to the speed of light in the medium $v_{\text{medium}}$:
$$n_{\text{substance}} = \frac{v_{\text{vacuum}}}{v_{\text{medium}}}$$
The refractive index depends on the temperature T and wavelength λ of light and is expressed as $n_{\lambda}^T$. Refractometric standard conditions are a wavelength of λ= 589 nm (sodium D-line) and a temperature of
T = 20°C. The refractive index for these conditions is commonly written as $n_{D}$ or nD.
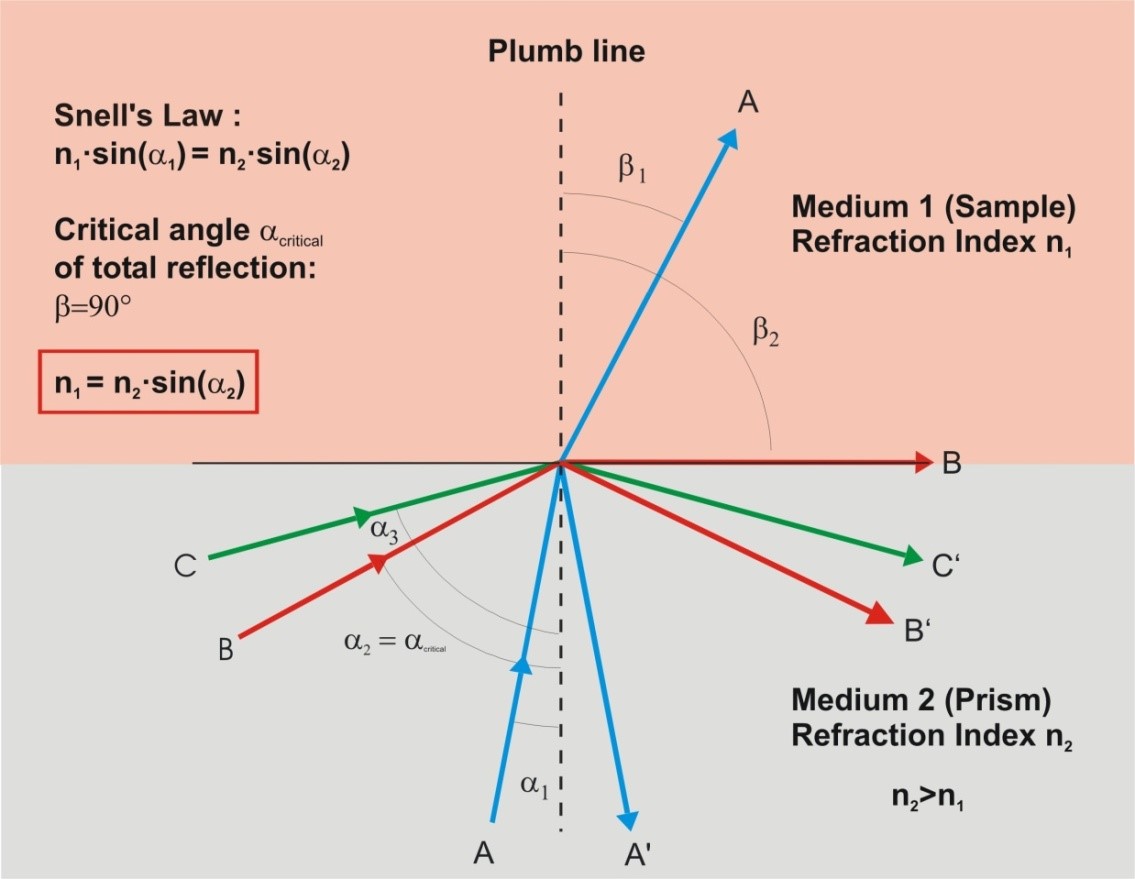
The change in speed at the interface between two media also causes a change of direction of the light beam, like waves on the shore. The amount of deflection depends on the difference of the refractive indices of the two media. At the interface not only refraction (change of speed and change of direction) takes place, but also reflection. The refractive index can be determined by measurement of the light reflection at the interface between two media of different optical densities.
The refraction of light at the interface of two media can be described by Snell’s law.
To measure the refractive index of an unknown sample, the critical angle of total reflection at the boundary surface of the sample and a prism is determined.
Ph. Eur. 2.2.6. methodology for measuring refractive index
Sample preparation
Unless otherwise stated in the monograph of the substance being measured, 1 mL to 2 mL of the sample is placed on the measuring surface of the refractometer. This ensures that the prism is fully covered, avoiding the introduction of air gaps that can affect the accuracy of the measurement.
Temperature and wavelength control
The refractive index must be determined at a controlled temperature of 20 ± 0.5 °C with reference to the wavelength of the D-Line of sodium (λ=589.3 nm).
Calibration
The refractometer requires calibration using certified reference materials to ensure accurate and reliable measurements. Calibration should be validated regularly according to the instrument's usage frequency and manufacturer’s recommendations.
Resolution and accuracy
Devices must provide readings accurate to at least the third decimal place. Instruments that use white light should be equipped with a compensating system to adjust for the dispersion effects caused by different wavelengths in the light source.
Results expression
The results are expressed with the symbol $n^{20}_D$, indicating that the refractive index is measured at 20 °C using the wavelength of 589.3 nm (with reference to the D-line of sodium). This standard notation helps maintain consistency in reporting across various studies and publications.
Deviations
Any deviations from these conditions are detailed in the specific monograph of the substance being tested. This might include adjustments to the temperature, wavelength, or specific handling procedures necessary due to the unique characteristics of the substance.
Incorporating these details ensures that the methodology section aligns with the specifications outlined in the European Pharmacopoeia 2.2.6, providing a clear and comprehensive guide for measuring refractive index under standardized conditions. This consistency is crucial for maintaining the quality and reliability of pharmaceutical testing and quality control processes.
Applications in pharmaceutical analysis
Substances requiring refractive index measurement in the European Pharmacopeia
The majority of substances included in the European Pharmacopoeia are essential oils, which are utilized in a multitude of products derived from botanically defined herbal drugs. A monograph published by the European Pharmacopoeia lists an essential oil as a component of a medicinal product, provided that it complies with the prescribed limits for the refractive index test and undergoes the requisite tests.
- Anise oil
- Benzyl alcohol
- Benzyl benzoate
- Bitter-fennel fruit oil
- Bitter-fennel herb oil
- Caraway oil
- Cassia oil
- Cineole
- Cinnamon bark oil, Ceylon
- Cinnamon leaf oil, Ceylon
- Citronella oil
- Clary sage oil
- Clofibrate
- Clove oil
- Cod-liver oil
- Coriander oil
- Crotamiton
- Cypress oil
- Dibutyl phthalate
- Diethyl phthalate
- Diethylene glycol monoethyl ether
- Dimercaprol
- Dimethyl sulfoxide
- Dimethylacetamide
- Dwarf pine oil
- Eucalyptus oil
- Juniper oil
- Lavender oil
- Lemon oil
- Mandarin oil
- Mint oil, partly dementholised
- Neroli oil
- Niaouli oil, cineole type
- Nutmeg oil
- Peppermint oil
- Petroleum rectificatum for homoeopathic preparations
- Pine sylvestris oil
- Rosemary oil
- Saw palmetto extract
- Spanish sage oil
- Spike lavender oil
- Star anise oil
- Sweet orange oil
- Tea tree oil
- Thyme oil, thymol type
- Turpentine oil
Equipment and techniques for refractive index measurement according to Ph. Eur. 2.2.6
Anton Paar’s Abbemat refractometers are designed to measure the refractive index accurately, fulfilling the stringent requirements set by the European Pharmacopoeia 2.2.6.
Calibration and compliance
The Abbemat refractometers are calibrated with certified reference materials to meet the Ph. Eur. standards, which require readings accurate to at least the third decimal place. These instruments comply with 21 CFR Part 11 and other regulatory standards, including Good Manufacturing Practices (GMP) and USP <1058>, supporting audit trails, electronic signatures, and user management systems necessary for regulatory compliance.
Equipped with internal Peltier elements for temperature stabilization, these refractometers manage the critical aspect of temperature influence on refractive index measurements. The precise temperature control ensures consistent and repeatable results, aligning with Ph. Eur. specifications. The measuring temperature can be checked, validated, and adjusted, if necessary, by the patented T-Check from Anton Paar.
Software features
The software integrated into Abbemat refractometers comply with pharmaceutical regulations, e.g. USP <831>, meeting 21 CFR Part 11, GAMP 5, and EU GMP Vol. 4 Annex 11. It offers secure data management, audit trails, electronic signatures, and access controls. The AISQ+ qualification package speeds up validation by 70 %.
The optional AP Connect lab execution software is a server-client solution that collects and archives data from Anton Paar and other instrument manufacturers to establish a vendor-independent data repository, create a unified interface to your LIMS, and fulfill regulative needs.
Adaptability and integration
Anton Paar refractometers can be integrated with other instruments to measure additional parameters such as density and viscosity, providing a comprehensive analysis capability that is beneficial for pharmaceutical laboratories looking to streamline multiple tests.
These refractometers, with their advanced technology and compliance features, are suitably equipped to perform refractive index measurements that strictly adhere to the conditions and requirements prescribed by the European Pharmacopoeia 2.2.6.
FAQs
1. What is the refractive index used for in pharmaceuticals?
The refractive index measurement contributes to the substance testing in regard to:
- Identifying a pure substance
- Establishing the purity of some substances
- Determining the concentrations of solutions such as alcohol solutions or sugar solutions (e.g. percentage of sugar in syrup or concentration determination of solvents)
- Determining the uniformity of final compounded preparations
2. How do environmental factors affect refractive index measurements?
The refractive index varies for nearly all materials with the wavelength of light. This so-called dispersion relation is characteristic for every material. The standard wavelength is the wavelength of the D-line of sodium, 589.3 nm.
Temperature is one of the major influences on the refractive index. Therefore, the temperature of the measuring prism and the temperature of the sample have to be controlled and measured with high precision. With the Abbemat T-Check, Anton Paar is in a position to check and adjust the temperature sensors of the Abbemat refractometers with high precision in the field to ensure accurate refractive index measurements with minimum downtimes at-site. Additionally, the requirement of pharmaceutical customers during the qualification process is fulfilled by proving not only the refractive index but also temperature measurement with the Abbemat refractometers.
3. What are the specific requirements for refractive index measurements according to Ph. Eur. 2.2.6?
- Refractive index is measured at 20 ± 0.5 °C, temperature resolution is ≤0.5 °C
- Wavelength of the D-line of sodium (λ = 589.3 nm); the symbol is $n^{20}_D$
- Calibration of the refractometer using certified reference materials
- Readings accurate to at least the third decimal place
References
- European Pharmacopeia, chapter 2.2.6
- Basics of refractometry