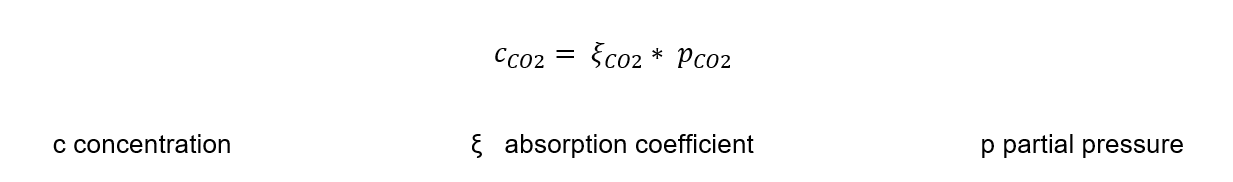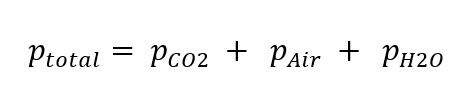Carbon dioxide in beverages
Introduction
In the world of beverage production, manufacturers share a common goal: ensuring top quality in their final packaged products. Quality control plays a crucial role in the operations of beverage producers, focusing on compliance, brand reputation, cost reduction, and continuous improvement.
Monitoring of carbon dioxide in beverages is crucial for quality control, especially in the final steps of the beverage production process. Whether in beer, soft drinks, mineral water, or sparkling wine, the carbon dioxide content must be monitored to produce and maintain the best possible quality and ensure high customer satisfaction.
Keeping an eye on the carbon dioxide level means measuring the amount of dissolved carbon dioxide gas in the liquid. Two common units are used for this purpose: volumes (vol.) and grams per liter (g/L). One volume represents 1 L CO2 dissolved in 1 L of beverage, which equals 1.96 g/L. (Ashurst, et al., 2017)
The packaged carbon dioxide originates mainly from the liquid phase. The beverages are carbonated either through industrial carbonators (e.g., carbonated soft drinks, mineral water, beer) or natural fermentation (e.g., beer, sparkling wine).
Carbon dioxide in beverages and other liquids
What is the role of carbon dioxide?
Carbon dioxide is a key quality parameter in beverage production, especially from the consumer point of view.
It gives the beverage its characteristic freshness and fizziness. Moreover, it improves the flavor and aroma. Carbon dioxide is also crucial for ensuring the taste of beer and the formation of the ideal foam head. Additionally, it acts as a preservative and exhibits oxidation-inhibiting effects, enhancing the shelf life and reducing microbiological growth within the beverage container. (Azeredo et al. 2016)
The carbon dioxide in beverages remains stable over a long period of time. Since carbon dioxide is a very stable, non-reactive gas it will not react with the packaging material or ingredients of the beverage itself. It does not get consumed or generated within the beverage container after filling. A decrease in carbon dioxide only occurs due to a loss to the surrounding atmosphere via the packaging material (e.g. PET) or the closure.
Measuring carbon dioxide
Fundamental gas laws
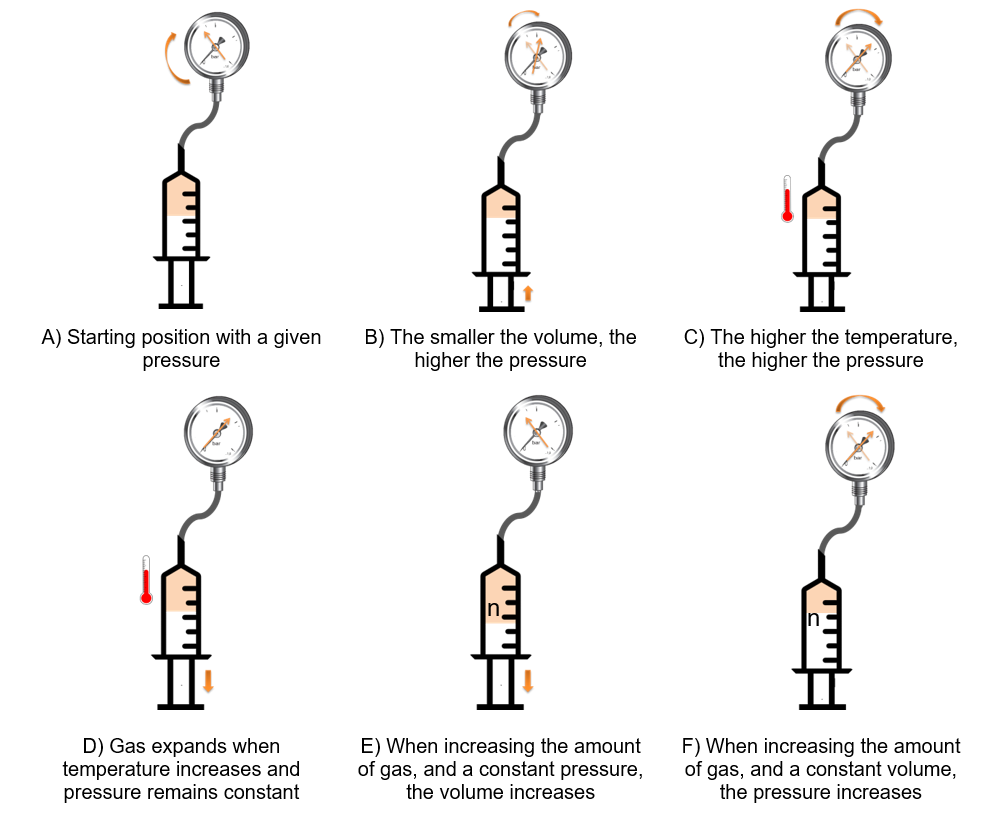
There are three fundamental gas laws which define the measurement of dissolved gases: In this case, CO2 in solution.
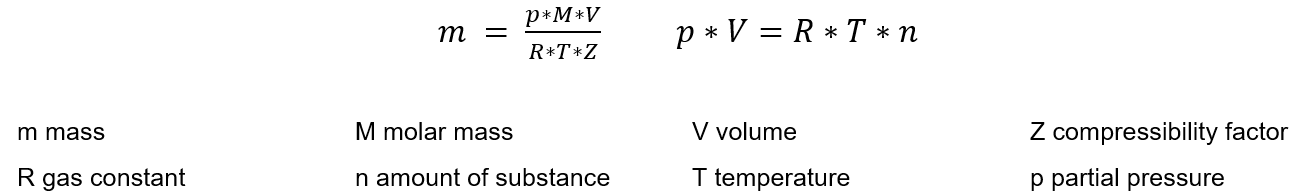
Ideal gas law
Henry’s law
Converting this gas law for the beverage industry, the carbon dioxide concentration in the beverage is proportional to the carbon dioxide partial pressure in the headspace of the beverage container. The respective solubility functions as the proportional factor.
Dalton’s law
The total pressure in a beverage container is created by the partial pressure of carbon dioxide, nitrogen and oxygen considered as air, as well as water vapors and to a small amount of other gases.
These gas laws are the basis for the following measurement methods.
Measurement methods
The concentration of carbon dioxide in beverages can be measured with different technologies. The most relevant are:
- Electrochemical method (Electrode, Severinghaus method)
- Physicochemical method (pressure/temperature method or multiple volume expansion method)
- Optical method (IR spectroscopy attenuated total reflectance method)
Electrodes based on the Severinghaus method are a low-cost alternative for the private sector and are not used in the beverage industry. Measurement devices based on the two physicochemical methods are commonly used in the quality control of the packaged product and in the production line. The multiple volume expansion method, on the other hand, allows beverage producers to selectively measure carbon dioxide in beverages, independent from other dissolved gases, like air or nitrogen.
However, conditions that contribute to accuracy must be considered, including proper standard operating procedure upon usage, sensor geometry and design, environment, and calibration/adjustment.
Multiple volume expansion method
Most process and laboratory instruments facilitate the classical p/T method of CO2 analysis according to Henry’s law, which defines the relationship between the concentration of the dissolved gas and its saturation pressure at a given temperature. The multiple volume expansion method is a redefinition of this method that adds a second volume expansion, using the fact that the solubility of CO2 in beverages is significantly higher than the solubility of other gases such as dissolved air, oxygen, or nitrogen.
More information can be found here: Multiple Volume Expansion method
Optical measuring method – IR spectroscopy
Measurement devices based on infrared (IR) absorption spectroscopy measure the carbon dioxide concentration in the head space of a translucent beverage container, like PET or glass bottles. It is a non-destructive technique, which means it does not require the transfer of liquid into a measuring chamber. Therefore, it can be utilized to perform shelf life tests over an extended period of time using the same bottle.
An IR beam is transmitted through the head space of the bottle. The amount of light reaching the detector is measured. Based on the detected absorption at a certain wavelength, the amount of CO2 in the headspace and the liquid can be calculated (under the assumption that an equilibrium was established prior to the measurement).
More information about the infrared spectrum of carbon dioxide can be found here: Infrared spectrum of carbon dioxide
Optical measuring method – Attenuated total reflectance method
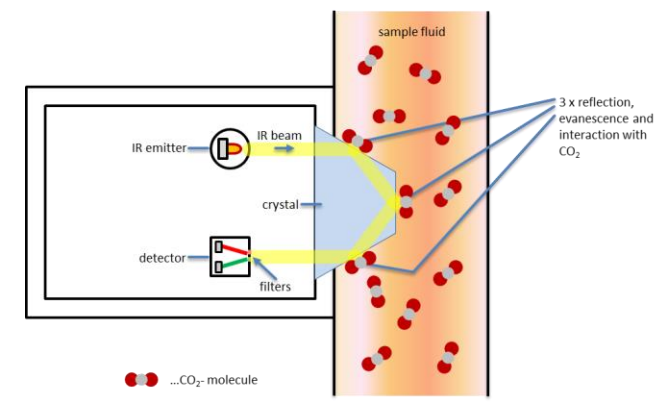
The attenuated total reflectance (ATR) method is mainly incorporated in a process sensor used in the production line. The method is based on the infrared light absorption at the surface of a crystal. The beam of infrared light passes through a crystal and is reflected at the internal surface in contact with the sample, as seen in Figure 2.
The infrared light absorption takes place in the surface layer of the sample on the crystal. The intensity of specific absorption bands of the reflected beam is measured by a detector and the corresponding CO2 value is calculated. As water and various beverage components also absorb light, specific reference measurements are necessary to accurately determine the CO2 content in beverages. The use of a reference beam allows the accurate calculation of carbon dioxide in beverages, independent of the beverage composition.
More information can be found here: Attenuated total reflectance (ATR)
Carbon dioxide monitoring points in the production process
Carbonation of the beverage is often one of the last steps on the way to the final packaged beverage. The following table summarizes the production steps of common carbonated beverages during which carbon dioxide concentrations are measured.
Table 1: Key steps including carbon dioxide measurement throughout common carbonated beverage production.
| Industry | Production step | Description | Impact |
| Carbonated soft drinks | Blending and carbonation | Various ingredients are mixed together in precise proportions. Carbon dioxide gas is dissolved into liquid. | Lack of carbon dioxide leads to reduced fizziness and potentially lower shelf life. Excess carbon dioxide causes unnecessary production costs. |
| Filling and packaging | Soft drink mixture is ready for packaging into bottles, cans and kegs. After filling, the package is sealed and labeled. | Poor filling causes loss of carbon dioxide, leading to out-of-specification production. | |
| Storage | Packaged products are stored in warehouses before distribution, during which CO2 loss needs to be prevented. | Poor container properties cause loss of carbon dioxide during storage, leading to out-of-specification products. | |
| Beer | Fermentation monitoring | Cooled wort is transferred to fermentation vessels where yeast is added. During fermentation, CO2 is produced by the added yeast. | An outside-of-range carbon dioxide level indicates a problem with the added yeast, and as a consequence, with the final alcohol content. |
| Beer blending | Beer batches are mixed (if necessary) to create a unified product and improved consumer perception. | Out-of-specification carbon dioxide causes, amongst other things, unwanted foaming and taste. | |
| Packaging | Beer is ready for packaging into bottles, cans and kegs. After filling, the package is sealed and labeled. | Poor filling causes loss of carbon dioxide, leading to out-of-specification production. | |
| Storage | Packaged beer is stored in warehouses before distribution, during which CO2 loss needs to be prevented. | Poor container properties cause loss of carbon dioxide during storage, leading to out-of-specification products. | |
| Sparkling wine | Storage and maturation | During the second fermentation of wine, carbon dioxide is produced by the added yeast and sugar. This process takes months. | Carbon dioxide out-of-specification causes an unwanted consumer experience. |
| Carbonation | Wine can also be carbonated via an industrial carbonator. | ||
| Blending and bottling | Sparkling wine is ready to be filled in corked or capped bottles and labeled. | Poor filling causes loss of carbon dioxide, leading to out-of-specification production. |
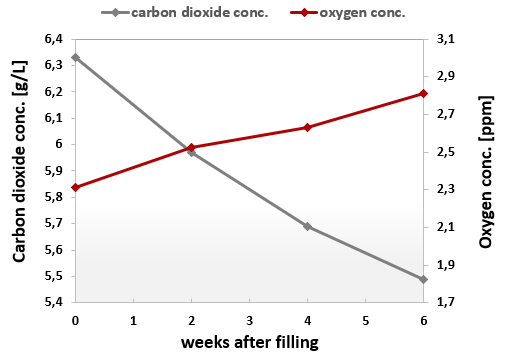
A significant factor for shelf life is the container which is chosen for the carbonated beverage. Whilst beer is mainly packaged in glass bottles and cans, roughly 70 % of soft drinks in Europe were packaged in PET bottles in 2020 (Unesda, 2024). Beverage cans do not allow oxygen ingress or carbon dioxide loss. Since glass has excellent gas barrier properties, the loss of carbon dioxide out of glass bottles depends on the closure type, crown cork or screwing cap (Jaime et al., 2022).
It is especially challenging for PET bottles to maintain the product-specific carbon dioxide level in the beverage container over the entire shelf life. Carbon dioxide diffuses out, while oxygen diffuses into the bottle. A selective dissolved carbon dioxide measurement is key to understanding this process because a decreased dissolved CO2 concentration results in a flat taste of the product and a higher risk of bacteria and mold growth (Azeredo et al. 2016). On the other hand, the slowly rising oxygen content may oxidize natural ingredients in the beverage, which finally influences the taste of the beverage.
Influences on carbon dioxide measurements
Instruments based on the multiple volume expansion method are less affected by outside factors compared to other techniques. Some factors which contribute to accuracy and reproducibility are summarized in the following table:
Table 2: Influencing factors on carbon dioxide measurement and their origin.
| Factor | What is causing the error? | Why? | |
| 1. | Calibration/ adjustment | Wrong adjustment | Faultily-adjusted measuring devices give wrong readings of pressure and temperature, leading to wrong carbon dioxide concentrations. |
| 2. | Low supply pressure at filling device | Bubbles in the measuring chamber | If bubbles are present in the measuring chamber after filling, the pressure reading is faulty and carbon dioxide data is inaccurate. |
| 3. | Sample preparation | Carbon dioxide distribution | Some techniques require a certain sample preparation in order to measure accurately. E.g., to measure dissolved carbon dioxide, it is necessary to shake the container 15 times horizontally in order to equilibrate the sample. |
| 4. | Sample composition | Carbon dioxide solubility | The solubility is a crucial factor in CO2 concentration calculation. Using a wrong solubility factor leads to an inaccurate measuring result. |
| 5. | Cleaning | Residues | Residues in the measuring chamber decrease the volume of the measuring chamber and increase the pressure reading accordingly. Additionally, the temperature and pressure sensor can be influenced by residues. |
Influencing factor: Sample preparation
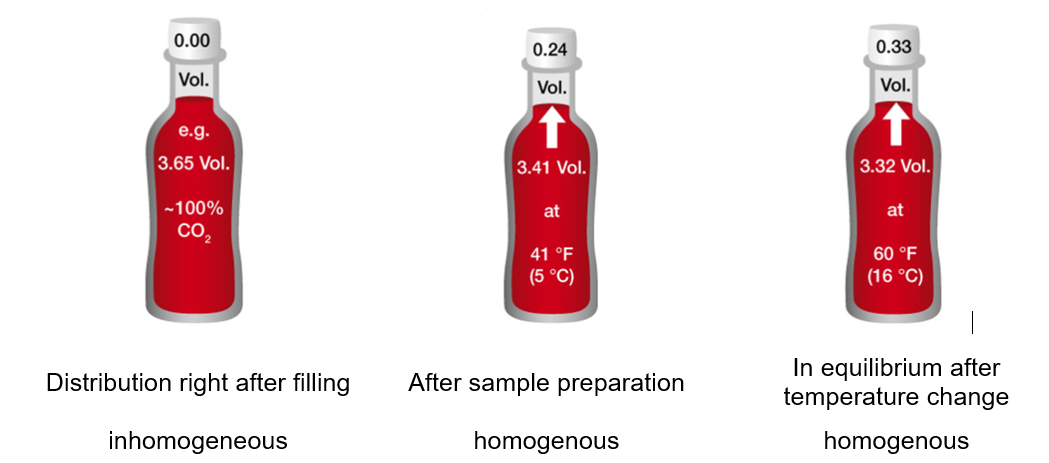
The overall content of carbon dioxide remains constant within an ideal closed beverage container. Depending on how the package was handled and whether/how the package was prepared, different levels of carbon dioxide can be expected. After filling the beverage container, almost no carbon dioxide is in the headspace. Right after filling, the CO2 starts to migrate into the headspace. The migration process is dependent on various factors, e.g. temperature and product composition. To generate repeatable and representative measuring results, repeatable conditions are a prerequisite. Therefore, generating an equilibrium between the headspace and the liquid phase by shaking is essential.
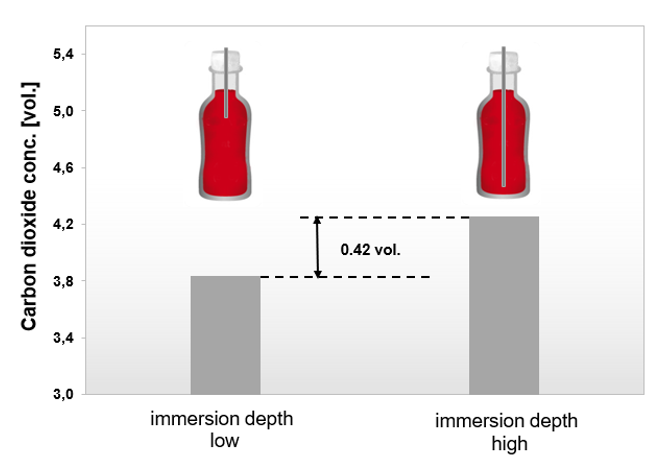
Measuring the CO2 concentration in an unshaken 1.5 L bottle of carbonated soft drink reveals the extent of the gradient within a beverage container:
The migration of carbon dioxide into the headspace starts at the liquid/gas-interface. The diffusion of the carbon dioxide within the liquid is slower than the migration into the headspace. Therefore, a gradient between the CO2 concentration at the top and the bottom of the liquid phase can be observed.
Additionally, the temperature of the measured sample plays an important role, as shown in Figure 4. The higher the temperature, the lower the ability of water to capture dissolved carbon dioxide. The CO2 will migrate into the headspace and alternates the dissolved CO2 concentration. To generate highly repeatable measurement results, tempering the beverage container should be part of the sample preparation (see Figure 4).
Influencing factor: Sample composition
The solubility of carbon dioxide in water is highly dependent on other dissolved components, like saccharose, high fructose corn syrup, alcohol or flavorings, as well as on temperature and pressure. The higher the content of sugar and/or oxygen, the lower the solubility of carbon dioxide. Since the solubility is, based on Henry’s law, a proportional factor in the calculation of the CO2 concentration, the correct solubility has to be used. In Table 3, a compilation of dissolved components and calculated carbon dioxide is shown. Using the wrong solubility factor can lead to an error up to 0.4 g/L (0.2 vol.).
Table 3: Compilation of beverage types, solubility-determining component and CO2 concentration. Mineral water sample was measured at 23.7 °C with a CboxQC At-line and results converted to different beverage types.
| Beverage type | Solubility-determining beverage component | CO2 conc. [g/L] |
| Mineral water | 0 ° Brix | 6.13 |
| Diet drinks | 0 °Brix to 3 °Brix | 6.06 |
| Medium-sugar beverages | 3 °Brix to 8 °Brix | 5.89 |
| High-sugar beverages | 8 °Brix to 12 °Brix | 5.73 |
| Beer | 10 °Plato to 14 °Plato | 5.90 |
| Strong beer | > 14 °Plato | 5.78 |
| Sparkling wine | 9 % v/v to 13 % v/v | 5.72 |
Dissolved carbon dioxide influences other measuring parameters as well. The sugar content and the apparent extract in beer, in particular, depend on the CO2 concentration of the sample. Both parameters are calculated based on the density of the beverage. Ideally, the CO2 content and the density are measured simultaneously. First measuring the CO2 content, followed by degassing and density determination, will also lead to the desired measurement results.
The number of dissolved components, like sugar, flavorings, acids and alcohol, are crucial quality parameters in beverages. The physical parameters - namely density and/or refractive index - can be used to calculate a total amount of all dissolved solids and liquids. In terms of soft drinks, density is often used as the measuring parameter of choice. The dissolved carbon dioxide increases the density of the beverage. To calculate true sugar content, which includes all dissolved components, a CO2-corrected density has to be used. To illustrate the influence of dissolved carbon dioxide in the beverages on its density, Table 4 shows the measured density, the CO2-corrected density, the carbon dioxide level, and corresponding calculated sugar contents.
Table 4: Measured density, the CO2-corrected density, the carbon dioxide level and corresponding calculated sugar content for common soft drink at 23 °C.
| Beverage type | CO2 conc. [g/L] | Density [g/cm³] | Sugar content [°brix] | CO2-corrected density [g/cm³] | CO2-corr. sugar content [°brix] | Difference in sugar content [°brix] |
| Mineral water | 6.10 | 1.00029 | 0.54 | 0.99889 | 0.18 | 0.30 |
| Wellness drink | 3.35 | 1.01048 | 3.15 | 1.00976 | 2.97 | 0.19 |
| Lemon-flavored soft drink | 4.55 | 1.03102 | 8.27 | 1.03010 | 8.04 | 0.23 |
| Orange-flavored soft drink | 4.00 | 1.04105 | 10.71 | 1.04028 | 10.52 | 0.18 |
| Cola | 6.31 | 1.04170 | 10.87 | 1.04044 | 10.56 | 0.36 |
Applications
In addition to the typical applications that mainly measure dissolved carbon dioxide concentration in-line, at-line or in the laboratory environment within the beverage industry (e.g., soft drinks, beer and wine), there is a constant need for carbon dioxide measurements in various other applications.
The demand for proper carbon dioxide measurements is increasing, due to climate change and the need to find processes to prevent, convert or store industrially generated carbon dioxide. Besides others, research activities on storing carbon dioxide in the seafloor require an accurate carbon dioxide measurement in saline water to calculate an escape rate of the hidden carbon dioxide (Flohr et al., 2021).
Furthermore, industries and institutions working with living marine species require a continuous carbon dioxide measurement in water to guarantee proper living conditions. Those industries and institutions include:
- Aquariums for public and private use
- Research institutions
- Fish and mussel farming
Carbon dioxide measurements in the gas phase are widely important in many industries and applications, like the automobile industry, plant-growing industry and air quality measurements.
Conclusion
In quality control, the measurement of dissolved carbon dioxide in beverages is a fundamental requirement. This critical parameter holds significant importance due to the substantial impact on the final product and its shelf life stability.
Through effective control of this variable, beverage producers can uphold superior quality standards, reduce costs and consistently meet customers’ expectations. Carbonization and packaging processes can be optimized and errors immediately detected during production by continuous monitoring of dissolved carbon dioxide.
Manufacturers are investing in instruments that enable selective, accurate and precise measurements to meet requirements and demands. State-of-the-art technologies like the multiple volume expansion method or attenuated total reflectance method are indispensable for achieving the necessary accuracy and precision.
Such sensors can be used in-line, at-line or in the laboratory environment, providing a comprehensive overview across the entire production line.
For more information about oxygen in beverages, see the corresponding Anton Paar wiki article: Oxygen in beverages.