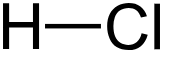Measuring the density of acids
Acids have a multitude of uses in different industries including the chemical, cleaning, food, metal, mining, petrochemical, pharmaceutical and textile industries. These sectors highlight the versatility and importance of acids in various industrial applications, making them critical chemicals in numerous manufacturing and processing activities.
Determining the concentration of acids is essential for optimizing performance and safety in these applications, with options including acid-base titration or the more efficient method of measuring.
Table 1 contains a partial list of common acids, along with their chemical formulas and applications.
Table 1: Common acids and their applications
| Chemical name | Chemical formula | Applications |
| Hydrochloric acid | HCl | Metal refining, pickling of steel, production of metal chlorides, pH control of process water and neutralization of waste streams, household cleaning, regeneration of ion exchangers (ChemicalSafetyFacts, 2022) |
| Sulfuric acid | H2SO4 | In car batteries, manufacture of fertilizers, production of detergents, synthetic resins, dyestuffs, pharmaceuticals, petroleum catalysts, and insecticides (The Editors of Encyclopaedia Britannica, 2024) |
| Nitric acid | HNO3 | Manufacture of fertilizers, explosives, nylon precursors and organic compounds, as a rocket propellant, during metal processing, gold parting (PubChem, no date) |
| Phosphoric acid | H3PO4 | Manufacture of fertilizers, acidification of foods and beverages, as a preservative, for chemical polishing of aluminum and steel passivation, as a sanitizing agent in the dairy, food and brewing industries (PubChem, no date) |
| Acetic acid | CH3COOH | Chemical reagent for the production of vinyl acetate, esters and acetic anhydrate, as a solvent and vinegar (Brown, 2024) |
| Hydrofluoric acid | HF | Production of organofluorine compounds and inorganic fluorides, as an etchant in the semiconductor industry (CDC, 2018) |
Why measure the density of acids?
Acids of defined concentrations are commonly needed for important reasons related to safety, cost efficiency, chemical attributes, and practical requirements.
- Reduced hazard/handling: Concentrated acids are highly corrosive and can damage materials and harm living things. Diluted acids are safer to transport, store, and less hazardous to handle, reducing the risk of accidents.
- Control of reaction rate: Diluted acids react more slowly, which is important to avoid side reactions and to maintain control over chemical processes, reducing the risk of violent exothermic reactions, which can lead to overheating and potentially dangerous situations.
- Specific concentrations: Many chemical processes and industrial applications require acids at specific concentrations. Dilution allows for achieving the exact concentrations needed for a particular application (Table 1).
- Economical: In many cases, it is more economical to dilute concentrated acids rather than purchasing and storing large quantities of pre-diluted acid.
Therefore, it is crucial to quickly, easily, and accurately determine the density of acids of both diluted and concentrated samples. A modern digital density meter combines these qualities in a single device.
Diluted acids typically consist of a binary system of the respective acid and water as the solvent. Using an appropriate concentration table, or more generally, a conversion formula, the measured acid density can easily be converted into the corresponding acid concentration.
What to consider when measuring acids using a digital density meter?
There are some considerations to keep in mind when measuring the density of acids using a digital density meter. Most modern laboratory density meters are equipped with a measuring cell made of borosilicate glass 3.3, which is chemically resistant against most diluted and concentrated acids and even their mixtures. However, its chemical stability is not only connected to the concentration of a given substance, but also to temperature and contact time.
- Corrosive samples should be left inside the cell for as short a time as possible and proper cleaning has to be performed immediately after the measurement.
- As higher temperatures can increase the corrosion rate, lower cell temperatures are generally recommended.
- Regular instrument checks (e.g. with distilled water) can detect problems with corrosion and associated surface abrasion of the cell wall, resulting in higher density readings in the water check.
- If corrosion cannot be prevented, an adjustment can be made to compensate for the abrasion of the cell wall over time, ensuring accurate results are maintained.
- In addition to the measuring cell, other wetted parts must also be checked for their resistance to the sample.
Hydrochloric acid
Hydrochloric acid (HCl) is a strong, inorganic (= mineral) acid. Its physical properties, including density, depend on the concentration or molarity of HCl in the aqueous solution, which range from those of water at very low concentrations approaching 0 % HCl to values for fuming HCl higher than 40 % HCl. This concentration range can be accurately measured using a digital density meter with a cell out of borosilicate glass 3.3.
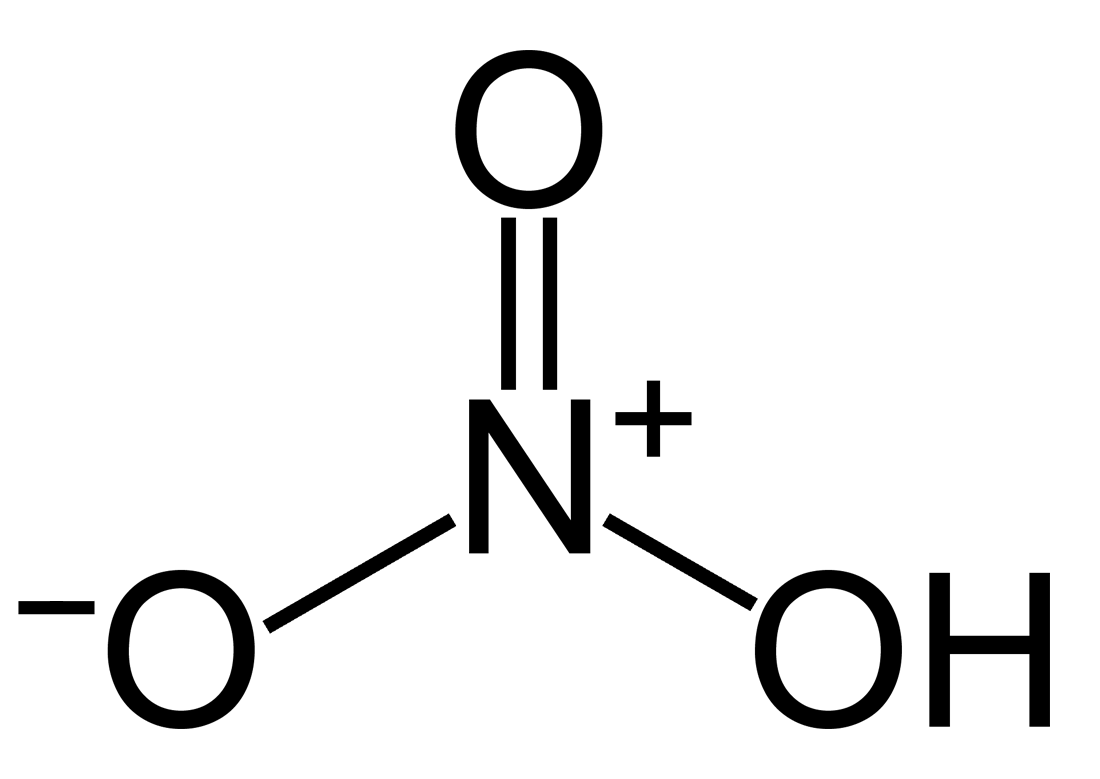
Nitric acid
Most commercially available nitric acid (HNO3) has a concentration of approximately 68 % to 70 % in water (concentrated grade). When the solution contains more than 86 % HNO3, it is referred to as fuming nitric acid. There are two types based on the presence of nitrogen dioxide (NO2): white fuming nitric acid, which contains only small amounts of NO2 and appears colorless and red fuming nitric acid, which contains a significant amount of NO2, giving it a red or yellow color. Analogous to HCl, the entire concentration range of interest can be covered with a digital density meter with a cell made out of borosilicate glass 3.3.
Hydrofluoric acid
Hydrofluoric acid (HF) is commonly manufactured by diluting 100 % hydrogen fluoride with water, and normally sold to industry in concentrations of 49 % HF and 70 % HF. Although HF is regarded as a weak acid in dilute aqueous solutions, it is very corrosive, even attacking glass when hydrated. Although borosilicate glass 3.3 is not resistant to HF even at low concentrations, a measurement using a digital density meter is possible when equipped with a measuring cell made of a metal alloy (e.g. Hastelloy C-276, a nickel-molybdenum-chromium alloy with tungsten). Since surface abrasion is also to be expected even with a cell made of metal, temperatures should be kept as low as possible and contact time as short as possible. Measurements of HF are possible up to a concentration of 40 % HF at 20 °C.
Note: Depending on the acid or acid mixture, cells made of borosilicate glass or metal alloys may offer better resistance.
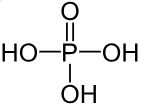
Phosphoric acid
Phosphoric acid (H3PO4) is commercially available as aqueous solutions of various concentrations, usually not exceeding 85 %. Borosilicate glass 3.3 exhibits limited stability against phosphoric acid, especially when hot. Therefore, hot phosphoric acid must not be filled in the cell, but at moderate temperatures (20 °C) measurements up to approximately 40 % H3PO4 are possible.
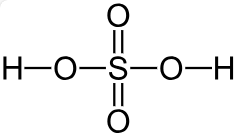
Sulfuric acid and oleum
Sulfuric acid (H2SO4) is a colorless, odorless and viscous mineral acid which can be diluted with water in any ratio. In its pure form it is highly hygroscopic, readily absorbing water vapor from the air. H2SO4 with 98.3 % is typically described as concentrated sulfuric acid, and several lower concentrations are commonly used depending on the application (e.g. 29 % to 32 % as battery acid).
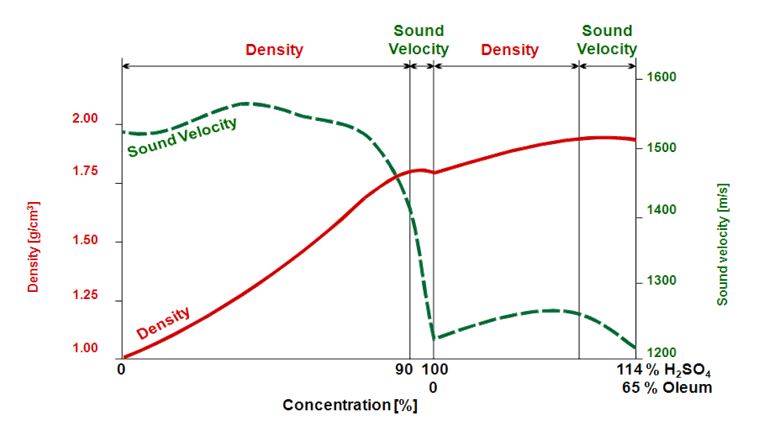
Fuming sulfuric acid, or oleum, is a term that refers to solutions of sulfur trioxide in sulfuric acid, which effectively extends the concentration range beyond 100 % when considered as sulfuric acid (0 % to 65 % oleum corresponds to 100 % to 114 % sulfuric acid) and it exhibits another interesting characteristic. Density and concentration do not show a linear relationship over the entire range. Sulfuric acid can be measured up to a concentration of 90 % via density alone. At this concentration the density value shows a maximum, and higher concentrations can no longer be correlated to one concentration only (Figure 1). Therefore, another parameter, e.g. sound velocity, must be measured to cover the entire concentration range. Devices that can measure both density and sound velocity in combination are available on the market. However, if this high concentration range is not of relevance, a digital density meter with a borosilicate glass cell is sufficient.
Mixtures of oleum or concentrated sulfuric acid with water or other solvents can cause very strong exothermic reactions, which may destroy the digital density meter and/or cause serious injuries.
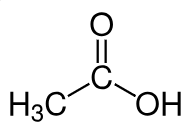
Acetic acid
Acetic acid (CH3COOH) is an organic compound and a colorless liquid. Its anhydrous form is referred to as glacial acetic acid. As with sulfuric acid, there is no linear relationship between density and concentration over the entire range from 0 % to 100 %. Density can cover the range between 0 % to 60 %, but beyond that, an additional parameter such as sound velocity is required.
Advantages of measuring density of acids in comparison to acid-base titration
To determine the concentration of a given acid, acid-base titration is the classical approach. However, using a digital density meter offers several advantages.
Density meters provide faster results, require less preparation and fewer chemicals than a titration. They are often automated, require less manual intervention, and can deliver very precise and reproducible measurements. Furthermore, they are not dependent on pH indicators used in titrations, which can lead to inaccurate results. Handling is safer and more environmentally friendly: Laboratory staff have less contact with corrosive or hazardous substances and density meters can be used for a variety of acids and concentrations without the need to develop specific titration methods.
Furthermore, modern devices often offer temperature compensation to ensure measurement accuracy at different temperatures.
Conclusion
The use of digital density meters significantly enhances the accuracy and efficiency of measuring acid concentrations in various industrial applications. These instruments provide a practical and reliable alternative to traditional acid-base titration, minimizing preparation time, reducing chemical use, and improving safety.
Digital density meters are particularly advantageous for handling corrosive acids, offering automated and precise measurements that ensure consistent and reproducible results. Their versatility in accommodating different acid types and concentrations, coupled with features like temperature compensation and sound velocity measurement for highly concentrated acids, makes them an essential tool in modern laboratories.
In summary, digital density meters not only streamline the process of acid concentration measurement but also contribute to safer and more sustainable laboratory practices, making them indispensable in both research and industrial settings.
References
Brown, W. H., 2024. Acetic acid. [Online]
Available at: www.britannica.com/science/acetic-acid
[Accessed 24 June 2024].
CDC, 2018. Facts About Hydrogen Fluoride (Hydrofluoric Acid). [Online]
Available at: emergency.cdc.gov/agent/hydrofluoricacid/basics/facts.asp
[Accessed 24 June 2024].
ChemicalSafetyFacts, 2022. Hydrochloric acid. [Online]
Available at: www.chemicalsafetyfacts.org/chemicals/hydrochloric-acid/
[Accessed 24 June 2024].
PubChem, n.d. Nitric acid. [Online]
Available at: pubchem.ncbi.nlm.nih.gov/compound/Nitric-Acid
[Accessed 24 June 2024].
PubChem, n.d. Phosphoric acid. [Online]
Available at: pubchem.ncbi.nlm.nih.gov/compound/Phosphoric-Acid
[Accessed 24 June 2024].
The Editors of Encyclopaedia Britannica, 2024. Sulfuric acid. [Online]
Available at: www.britannica.com/science/sulfuric-acid
[Accessed 24 June 2024].