2D materials

Single-layer materials are the focus of research for very versatile applications. These include nano-sized strain gauges, nanocrystalline TiO2 coatings for body implants, or, for example, the study of 2D material crystallinity of the anode or cathode component for faster and more efficient energy transfer in batteries.
In this section, we show how various measurement solutions and different technologies from Anton Paar play an important role in the characterization of 2D materials such as temperature-controlled grazing incidence, small-angle X-ray scattering (GISAXS) to study the thermal properties of self-assembled thin-film structures, surface zeta potential to study protein adsorption on TiO2 anatase coatings, or use of gas pycnometry to measure skeletal density of a series of graphenes with varying crystallinity.
Characterization of 2D nanostructures of self-assembled gold nanoparticles
Introduction
Colloidal nanoparticles are widely used due to their specific electronic, chemical and biological properties. E.g. colloidal gold (Au) may form two- and three-dimensional structures comprising monolayers of hexagonally-packed Au nanoparticles. Such materials are promising systems for, e.g. nano-sized strain gauges. The applicability of such thin-film materials may of course be limited by the mechanical or thermal stability. Hence, an in-situ method like temperature-controlled grazing-incidence small-angle X-ray scattering (GISAXS) is an ideal tool for studying the thermal properties of such self-assembled thin-film structures.
Experimental
A homogeneous layer of hexagonally arranged Au nanoparticles was deposited on Si wafer substrates by using a Langmuir-Schaefer method. The obtained thin films were measured with an Anton Paar SAXSpoint system equipped with a dedicated GISAXS stage and heating module. Structural changes of the sample were monitored from ambient temperature up to 220 °C. For precise evaluation of GISAXS scattering patterns, BornAgain [1] is currently the most advanced software – it allows for simulation of GISAXS data. With a dedicated data export routine, GISAXS data acquired with the SAXSpoint system can directly be transferred to the BornAgain platform.
Results and discussion
At room temperature, the acquired GISAXS pattern showed the self-assembled Au monolayer to have a typical hexagonal close-packed (hcp) arrangement. In in-situ heating GISAXS experiments, this structure was found to be stable to temperatures of up to 180 °C, proving the good stability of this system. Above 180 °C, a transformation of the small Au nanoparticles to larger sizes was observed, resulting in a stable nanostructure above 195 °C. This proves that in-situ GISAXS is a valuable tool to monitor the structure, stability and structural changes of such nanosized 3D structures.
For detailed evaluation, the GISAXS patterns of the sample exhibiting hcp arrangements were automatically exported and analyzed with the BornAgain software. The fit calculated with regard to the experimental data revealed a particle size of around 6 nm for the Au nanoparticles, and a lattice constant of around 8 nm [2].


Additional information
Instruments:
- SAXSpoint
References:
- www.bornagainproject.org
- K. Vesgo, M. Jergel, P. Siffalovic, M. Kotlar, Y. Halahovets, M. Hodas, M. Pelletta, E. Majkova, Sensors and Actuators A: Physical 241 (2016) 87-95.
Application reports:
Protein adsorption on nanocrystalline TiO₂ coatings
Introduction
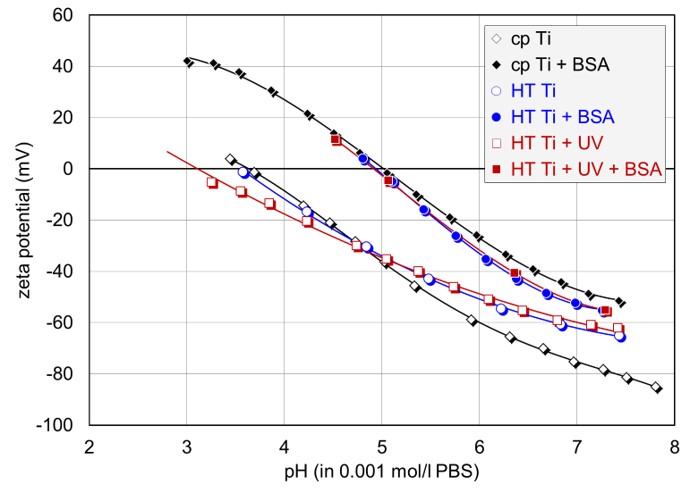
Ti-based materials are among the materials most commonly used for hard tissue replacement, as they provide suitable mechanical and chemical properties. This study focuses on the adsorption of proteins on TiO2 anatase coatings and the influence of UV irradiation prior to protein adsorption. The reported results give detailed insights into the surface zeta potential characterization of nanocrystalline TiO2 coatings for body implants, and thereby provide a basis for studies related to the hemocompatibility and biocompatibility of this material class.
Experimental
Commercially pure (cp) and hydrothermally treated (HT) TiO2 disks were used as a base material for conduction of zeta potential measurements and adsorption kinetic studies with a SurPASS. PBS served as an electrolyte solution. Ph titration curves were obtained using HCl as an acid and NaOH as a base. Bovine serum albumin (BSA) was used as a protein for investigation of time-resolved adsorption.
Results and discussion
All samples had an isoelectric point (IEP, pH value where the zeta potential is 0 mV and a charge reversal takes place) at acidic pH, and a negative zeta potential at physiological pH, with the highest negative zeta potential occuring for the cp sample. This observation can be related to the amorphous oxide structure of HT samples, which altered density and stability of surface hydroxyl groups.
The exposure to BSA led to a shift of the IEP to a pH value of around 5, confirming full coverage of all investigated TiO2 surface with the globular protein. Even though the shape of the titration curves did not allow any further conclusions on the adsorption behavior, the recoded adsorption kinetics provided deeper insights and revealed the fastest rate of adsorption for the UV pre-irratiated HT sample, with gradually lower rates down to the cp sample.

Additional information
Instruments:
Source:
S. Novak et al., Biomed. Mater. 10 (2015) 045012
Surface characterization of self-assembled monolayers deposited on gold substrates
Introduction
Self-assembled monolayers (SAMs) are spontaneously formed assemblies of organic molecules adsorbed on solid surfaces, and enable the control of their physiochemical properties. This type of nanomaterial is an attractive candidate for tailoring the surface properties of biological and biotechnological surfaces, like biosensors, as it doesn’t influence the sample thickness or the materials’ bulk electrical properties. This study focuses on the investigation of organic SAMs with different functionalities and the characterization of the surface zeta potential. This relationship can provide valuable information on the SAMs electrostatic interaction with nano-sized materials, and can trigger the adsorption and desorption of the latter.
Experimental
Investigated samples:
Thiol-based SAMs with pure COOH, pure NH2, and mixed functionality deposited on gold substrates.
Characterization technique:
Measurements of the surface zeta potential were conducted with the SurPASS electro-kinetic analyzer equipped with Ag/AgCl electrodes and the adjustable gap cell for planar samples. An electrolyte 0.1 mM NaCl solution was used. Ph titration curves were obtained by adding an acid (HCl) and a base (NaOH) to the electrolyte solution.
Results and discussion
Thiol-based SAMs with different ratios of amine and carboxyl terminal groups were characterized according to their surface zeta potential. The study of zeta potential versus pH value revealed a clear shift in the isoelectric point (IEP, pH value where the zeta potential is 0 mV and a charge reversal takes place) with changing ratio of functional groups. The higher the amine content, the further the IEP was shifted from acidic towards neutral pH. However, the increase in IEP was not linear with the ratio of terminal groups; still, the contribution of the COOH functionality dominated. This can be related to the fact that the carbon chain of COOH is longer and therefore more easily accessible compared to the chain of NH2 as depicted in Figure 2.


Additional information
Instruments:
Source:
*Shyue et al., Phys. Chem. Chem. Phys. 11 (2009) 6199-6204
Crystallinity of graphene and relation to battery electron transfer properties
Introduction
Improving electrode battery components to achieve faster and more efficient energy transfer is an important part of battery material research and design. In particular, crystallinity is a critical property of solid battery electrode components because it enables ions to conduct efficiently through the anode and cathode instead of slowing down in the amorphous domains. The higher the crystallinity of the anode or cathode component, the more efficient the electron transfer through and between the components. Here we use gas pycnometry to measure the skeletal density of a series of graphenes with varying crystallinity, where the higher skeletal density correlates to a more crystalline material.
Experimental
Characterization techniques: Skeletal density measurements of a series of graphenes were performed using an Anton Paar Ultrapyc 5000 gas pycnometer. Because of the porous nature of these carbons, the samples were vacuum degassed for 3 hours at room temperature on an external degasser before being transferred to the instrument sample cell. The samples were then conditioned inside the pycnometer using a flow of helium purge gas for 3 min. The gas expansions were performed using helium from a starting pressure of 1.17 bar.
Results and discussion
The skeletal densities for 3 different electrode carbons were found to be 2.117 g/cm3, 2.139 g/cm3, and 2.152 g/cm3. The data in the table below show that density can be measured with excellent repeatability, <1% for all samples. Comparing the 3 samples, the density increases from Sample A to Sample B to Sample C. It is known that increased crystallinity correlates with an increase in skeletal density, so Sample C was the most crystalline of these 3 samples of electrode carbon. The higher the crystallinity, the more efficient that material is for electron transfer, making Sample C also the best choice for a battery anode.
Sample | Density (g/cm3) | Repeatability (%) | |||
| Run 1 | Run 2 | Run 3 | Average |
|
A | 2.120 | 2.114 | 2.118 | 2.117 | 0.11 |
B | 2.138 | 2.139 | 2.139 | 2.139 | 0.02 |
C | 2.144 | 2.158 | 2.154 | 2.152 | 0.27 |
Additional information
Instruments:
Application report: